Introduction to Acidic Fibroblast Growth Factor
The Acidic Fibroblast Growth Factor (aFGF or FGF-1) is a protein, weighing approximately 16-kDa, that is found in numerous tissues. This protein exhibits a wide array of biological activities and is part of a group of at least nine known proteins with similar structure, collectively termed as fibroblast growth factors (FGFs). One of the distinguishing features of these proteins is their affinity towards sulfated polysaccharides like heparin and heparan proteoglycans, which are present on the majority of cell surfaces and essentially all basement membranes.
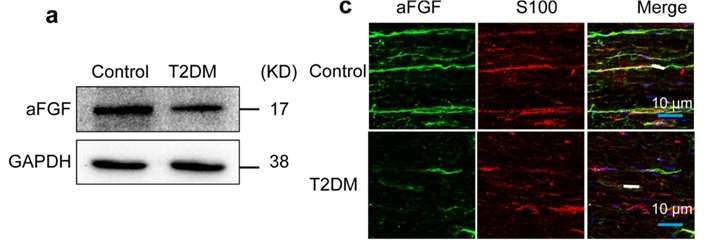 Fig 1. Endogenous levels of aFGF in T2DM mice and SCs. (Li, Rui, et al. 2021)
Fig 1. Endogenous levels of aFGF in T2DM mice and SCs. (Li, Rui, et al. 2021)
The FGF family includes two well-studied members, namely acidic (aFGF or FGF-1) and basic (bFGF or FGF-2) fibroblast growth factors. Both of these proteins serve as powerful mitogens for mesoderm-derived cells as well as several cells that originate from the embryonic ectoderm. Furthermore, aFGF and bFGF exert a chemotactic effect on cells in culture.
Turbidimetric Monitoring of aFGF Stability
When an aFGF solution is subjected to temperatures close to or greater than its transition midpoint (Tm) - the temperature at which the protein goes from a folded to unfolded state - the growth factor undergoes denaturation, losing its native tertiary structure. This denatured form of aFGF exhibits extreme insolubility and undergoes rapid aggregation as the unfolded protein emerges. Therefore, a convenient method to assess the structural stability of aFGF is to monitor the kinetics of temperature-induced aggregation by measuring the turbidity (degree of light scattering) at a wavelength of 350 nm.
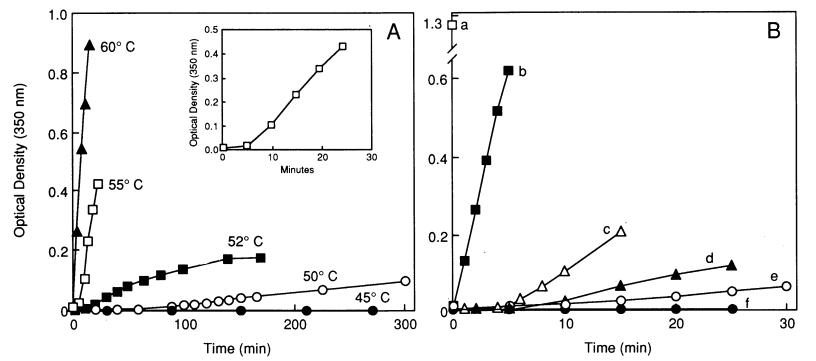 Fig 2. Turbidity measurements of the heat-induced aggregation of aFGF in the presence of heparin. (Tsai, P. K., et al. 1993)
Fig 2. Turbidity measurements of the heat-induced aggregation of aFGF in the presence of heparin. (Tsai, P. K., et al. 1993)
Delivery of aFGF as a Topical Agent
Developing aFGF into a therapeutic agent for wound healing involves more intricate considerations than merely ensuring its physicochemical and biological stability during storage. A key requisite involves the easy applicability of this biologically active growth factor to the wound in a clinical context, along with successful delivery and maintenance at the targeted tissue area.
To prevent run- off, a viscous preparation was considered appropriate provided it (1) does not interfere with bioactivity of the protein, (2) can withstand freezing at –70°C without loss of viscous properties, (3) is compatible with buffer components, (4) retains bioactivity of the protein after drying on the treatment area, (5) does not interfere with bioanalytical capabilities, and (6) can withstand sterilization.
Effect of Other Polyanions on aFGF Stability
To investigate the ability of polyanions other than heparin to stabilize acidic fibroblast growth factor (aFGF), a variety of composites analogous to heparin, including sulfated polysaccharides, sulfated and phosphorylated small molecules, and other strongly charged compounds, were investigated using turbidimetric methods at 40°C. The results of these investigations are summarized below. The ability of heparin analogs, such as sulfated polymers, to stabilize aFGF varies widely.
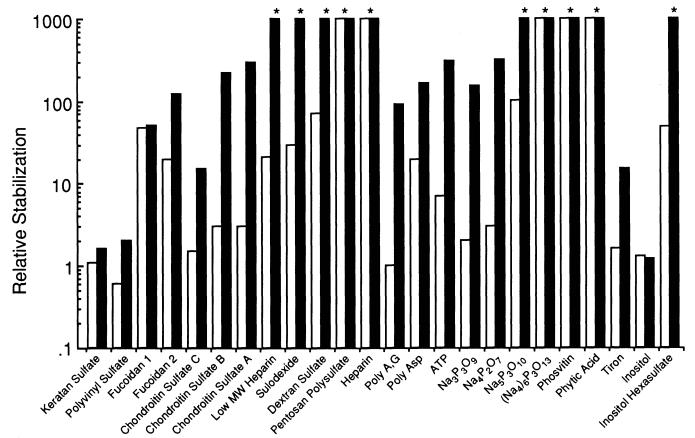 Fig 3. Stabilization by polyanions of aFGF against heat-induced aggregation at 40°C. (Tsai, P. K., et al. 1993)
Fig 3. Stabilization by polyanions of aFGF against heat-induced aggregation at 40°C. (Tsai, P. K., et al. 1993)
For example, heparin, pentosan sulfate, and dextran sulfate showed greater efficacy (no aggregates manifested after 30 minutes) compared with chondroitin sulfate (which showed 10- to 100-fold stabilization), which in turn showed greater efficacy (ranging from unstable to 1- to 10-fold stabilized) than either polyethylene sulfate or carrageenan sulfate.
What Can We Offer?
Creative Proteomics, a leading biotechnology company, utilizes state-of-the-art techniques and cutting-edge technologies to offer a wide range of comprehensive protein drug characterization services. With extensive expertise and a team of seasoned scientists, Creative Proteomics delivers accurate and reliable analyses, providing detailed insights into the characteristics, properties, and functions of protein drugs. Please feel free to contact us.
References
- Li, Rui, et al. Acidic fibroblast growth factor attenuates type 2 diabetes-induced demyelination via suppressing oxidative stress damage. Cell death & disease. 2021, 12.1: 107.
- Tsai, P. K., et al. Formulation design of acidic fibroblast growth factor. Pharmaceutical research. 1993, 10: 649-659.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. Endogenous levels of aFGF in T2DM mice and SCs. (Li, Rui, et al. 2021)
Fig 1. Endogenous levels of aFGF in T2DM mice and SCs. (Li, Rui, et al. 2021) Fig 2. Turbidity measurements of the heat-induced aggregation of aFGF in the presence of heparin. (Tsai, P. K., et al. 1993)
Fig 2. Turbidity measurements of the heat-induced aggregation of aFGF in the presence of heparin. (Tsai, P. K., et al. 1993) Fig 3. Stabilization by polyanions of aFGF against heat-induced aggregation at 40°C. (Tsai, P. K., et al. 1993)
Fig 3. Stabilization by polyanions of aFGF against heat-induced aggregation at 40°C. (Tsai, P. K., et al. 1993)