Introduction to recombinant human Relaxin
Relaxin, a protein hormone predominantly recognized for its significant function in the reproductive biology across various species. This unique protein orchestrates fluctuations in organ structure amid pregnancy and parturition by manipulating the reconfiguration of connective tissues within targeted organs. Moreover, relaxin is known to govern several biological responses in reproductive tissues in gestating animals, a topic we shall delve into, more extensively, in later sections.
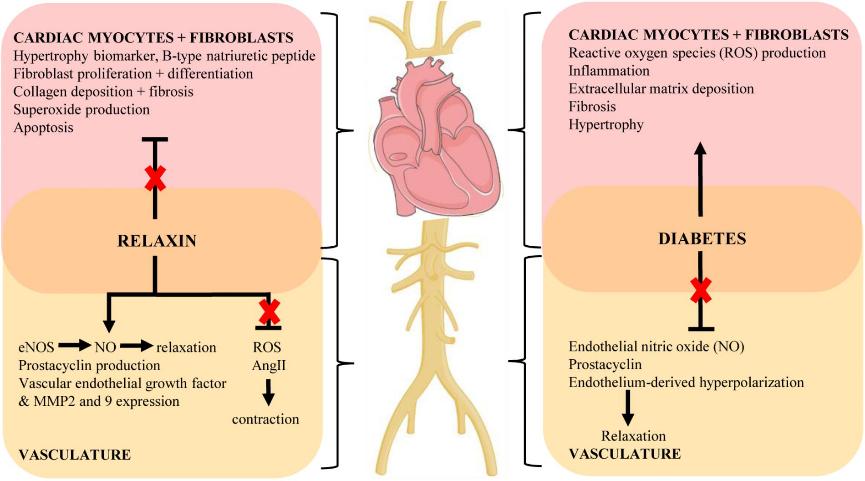 Fig 1. Key mechanisms of the cardiovascular complications of diabetes and proposed protective actions of relaxin to target these pathways (indicated by red cross). (Ng, H. H., et al. 2018)
Fig 1. Key mechanisms of the cardiovascular complications of diabetes and proposed protective actions of relaxin to target these pathways (indicated by red cross). (Ng, H. H., et al. 2018)
One of the potentially critical applications of relaxin as a therapeutic entity involves suppression of premature labor whilst inducing the process of cervical ripening prior to parturition. Numerous primitive and somewhat unregulated human clinical studies have been conducted, involving the use of porcine relaxin extracted from structures such as the corpus lutea and ovaries.
Primary Structure—Peptide Map
Recombinant human relaxin and its native relaxin obtained from ectopic pregnancy corpus luteum were chemically characterized using tryptic peptide mapping and mass spectrometry. Due to the limited number of native samples, it was not possible to perform a complete chemical analysis or mass collection of the entire relaxin molecule. This problem was addressed by calculating the masses of the individual A and B chains after reduction. The mass of the isolated native A chain matches the predicted mass of the cDNA-derived primary structure, taking into account the pyroglutamate amino terminus of the A chain.
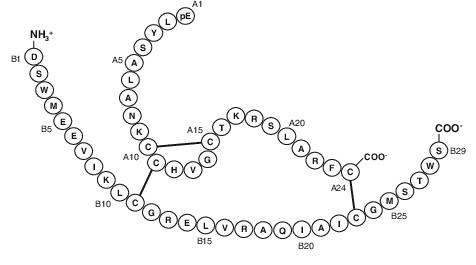 Fig 2. Structure of native and manufactured human relaxin. (Teichman, S. L., et al. 2008)
Fig 2. Structure of native and manufactured human relaxin. (Teichman, S. L., et al. 2008)
It is challenging to determine the actual size of the B chain because of the unpredictable protein hydrolysis processing sites in the procreatin sequence. By analogy with the processing sites of porcine and rat prorexins, the mass of the 33-amino acid B chain was predicted to be approximately 3798.6. However, the mass of the independent natural 29-amino acid B chain was detected to be significantly smaller at 3319.9.
Oxidation in the Presence of Light
Human relaxin is a protein with a B-chain length of 33 amino acids, which is degraded by strong light irradiation. This was achieved by applying reversed-phase high-performance liquid chromatography (RP-HPLC) and mass spectrometry.
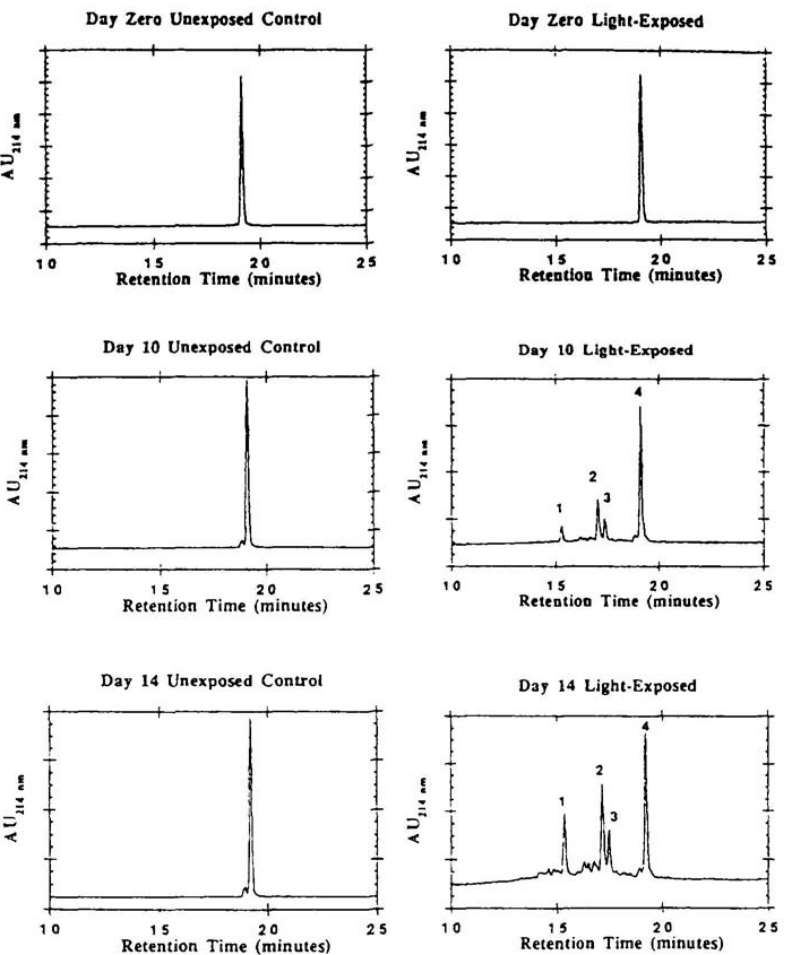 Fig 3. Reversed-phase HPLC analysis of synthetic human relaxin exposed to 3600 foot-candles of light for 0, 10, and 14 days. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Reversed-phase HPLC analysis of synthetic human relaxin exposed to 3600 foot-candles of light for 0, 10, and 14 days. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Upon exposure to light intensity equating to 3600 foot-candles for a duration of 17 days, the RP-HPLC analysis indicated notable alterations in the structure of relaxin. Further examination entailing a tryptic peptide analysis, executed both pre and post intense light exposure, documented alterations in the T6 and T9 tryptic peptides of the relaxin molecule.
What Can We Offer?
Armed with advanced technologies and a team of seasoned scientists, Creative Proteomics is capable of carrying out meticulous evaluations of elements such as protein drug structure, formulation, stability, and post-translational modifications. Moreover, to maintain their commitment to delivering high-caliber results, the company aligns strictly with stringent industry standards and regulatory guidelines. For more detailed exploration of our services, you are more than welcome to contact us.
References
- Ng, H. H., et al. Relaxin as a Therapeutic Target for the Cardiovascular Complications of Diabetes. Front Pharmacol. 2018; 9: 501.
- Teichman, S. L., et al. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Failure Reviews. 2008, 14(4), 321–329.
- Pearlman, Rodney, and Y. John Wang, eds. Formulation, characterization, and stability of protein drugs. Vol. 9. Springer Science & Business Media. 1996.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. Key mechanisms of the cardiovascular complications of diabetes and proposed protective actions of relaxin to target these pathways (indicated by red cross). (Ng, H. H., et al. 2018)
Fig 1. Key mechanisms of the cardiovascular complications of diabetes and proposed protective actions of relaxin to target these pathways (indicated by red cross). (Ng, H. H., et al. 2018) Fig 2. Structure of native and manufactured human relaxin. (Teichman, S. L., et al. 2008)
Fig 2. Structure of native and manufactured human relaxin. (Teichman, S. L., et al. 2008) Fig 3. Reversed-phase HPLC analysis of synthetic human relaxin exposed to 3600 foot-candles of light for 0, 10, and 14 days. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Reversed-phase HPLC analysis of synthetic human relaxin exposed to 3600 foot-candles of light for 0, 10, and 14 days. (Pearlman, Rodney, and Y. John Wang, eds. 1996)