Creative Proteomics offers protein isoelectric point (PI) determination services, which are useful in the study of protein structure, characteristics, and function, as well as in the manufacture and quality control of protein-related products.
Background
The protein isoelectric point is the pH at which a protein has no net electrical charge. It is the pH value at which the protein carries no positive or negative charge, and its net charge is zero. At the isoelectric point, a protein does not migrate in an electric field as it has no charge to move towards either the positive or negative pole. The pI of a protein is determined by the amino acid composition and the specific arrangement of charged and uncharged amino acid residues in the protein sequence.
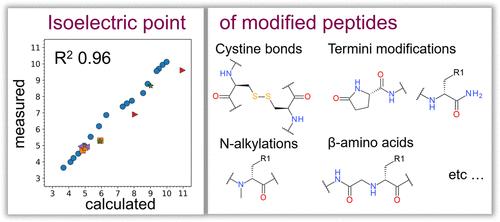 Fig 1. pIChemiSt ─ Free Tool for the Calculation of Isoelectric Points of Modified Peptides. (Andrey I., et al.; 2023)
Fig 1. pIChemiSt ─ Free Tool for the Calculation of Isoelectric Points of Modified Peptides. (Andrey I., et al.; 2023)
Zwitterions
Zwitterions (also known as dipolar ions) are molecules that have both a positive and a negative charge, but overall, they have no net electrical charge. In the context of proteins, zwitterions refer to the state of amino acids and proteins at their isoelectric point, where the positive and negative charges are balanced within the molecule.
What connection exists between an amino acid's pH and pKa?
The protonation-deprotonation equilibrium of the backbone amino group, backbone carboxyl group, and any potential acid/base component of the variable group is represented by the amino acid's pKa, which is an acid dissociation constant. The charge of each individual amino acid across the protein is added to determine the net charge of the protein.
The transition between an amino acid's protonated and deprotonated structural forms is defined by the equilibrium constant, or pKa. For the carbonyl group, amino group, and any functional groups on the side chain that may be protonated or deprotonated, amino acids have different pKas.
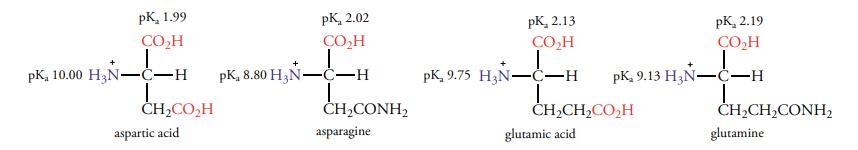 Fig 2. The difference between the pKa values of aspartic acid and asparagine is larger than the difference between the pKa values of glutamic acid and glutamine. (Ouellette, R. J., & Rawn, J. D.; 2015)
Fig 2. The difference between the pKa values of aspartic acid and asparagine is larger than the difference between the pKa values of glutamic acid and glutamine. (Ouellette, R. J., & Rawn, J. D.; 2015)
Because a carboxylate group and an amide group have different inductive effects on the ionization sites, the pKa values of the a-ammonium and a-carboxyl groups of aspartic acid and the structurally related asparagine differ. Due to the groups' greater distance from the ionization sites, the effect is less for glutamic acid than glutamine. The difference between the effects of the groups reduces until there is finally no effect when the chain between the groups and the reaction site lengthens.
What can we offer?
- This technique separates proteins based on their pI using a pH gradient gel. The gel is subjected to an electric field, and proteins migrate towards the pH at which they have no net charge, i.e., their pI.
Isoelectric focusing (IEF)
- Proteins can be separated based on their charge using a technique called gel electrophoresis. By running the proteins through a gel matrix at different pH values, proteins will move towards the electrode of opposite charge until they reach their pI.
- The titration curve of a protein involves determining the pH at which its net charge is zero. By titrating the protein solution with acid or base and monitoring the change in pH, the pI can be determined.
Why choose us?
Want to Learn More?
Customers all over the world may depend on Creative Proteomics for cost-efficient, high-quality, and hassle-free research solutions including protein drug characterisation. We promise to provide our products and outcomes on schedule. Feel free to get in touch with us.
References
- Andrey I., et al.; pIChemiSt ─ Free Tool for the Calculation of Isoelectric Points of Modified Peptides. Journal of Chemical Information and Modeling. 2023, 63, 1, 187–196.
- Ouellette, R. J., & Rawn, J. D. Amino Acids, Peptides, and Proteins. Organic Chemistry Study Guide. 2015, 569–586.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. pIChemiSt ─ Free Tool for the Calculation of Isoelectric Points of Modified Peptides. (Andrey I., et al.; 2023)
Fig 1. pIChemiSt ─ Free Tool for the Calculation of Isoelectric Points of Modified Peptides. (Andrey I., et al.; 2023)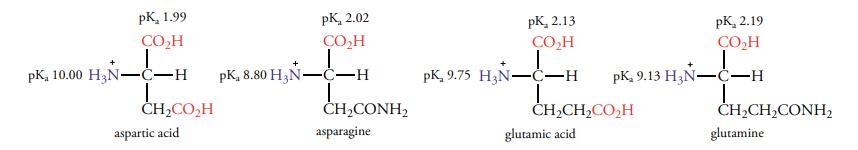 Fig 2. The difference between the pKa values of aspartic acid and asparagine is larger than the difference between the pKa values of glutamic acid and glutamine. (Ouellette, R. J., & Rawn, J. D.; 2015)
Fig 2. The difference between the pKa values of aspartic acid and asparagine is larger than the difference between the pKa values of glutamic acid and glutamine. (Ouellette, R. J., & Rawn, J. D.; 2015)



