ICH Topic Q6B states "Molecular variants resulting from changes in the desired product or product-related substances brought about over time and/or by the action of, e.g., light, temperature, pH, water, or by reaction with an excipient and/or the immediate container/closure system. Such changes may occur as a result of manufacture and/or storage (e.g., deamidation, oxidation, aggregation, proteolysis)."
Protein aggregation may lead to loss of protein activity, increase immunogenicity, and seriously affect protein quality indicators and production applications. Creative Proteomics provides protein aggregation analysis services, which play an important role in drug discovery and commercialization.
What is Protein Aggregation?
Protein aggregation refers to the process by which proteins form abnormally folded or misfolded structures, leading to the formation of aggregates or clumps. These aggregates can range in size from small oligomers to larger insoluble fibrils and amyloid plaques.
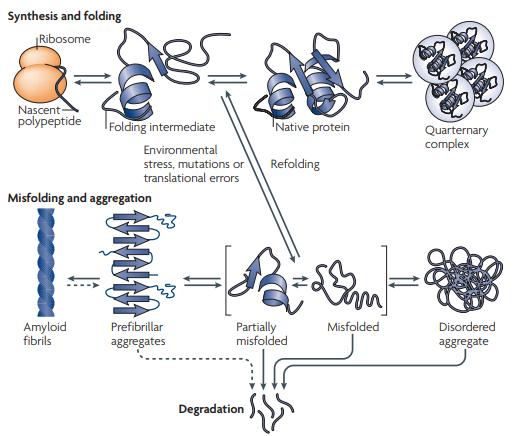 Fig 1. Overview of cellular protein aggregation. (Sani, E., et al.; 2010)
Fig 1. Overview of cellular protein aggregation. (Sani, E., et al.; 2010)
Protein aggregation can occur due to various factors, including genetic mutations, cellular stress, environmental changes, or aging. When proteins misfold, they can expose hydrophobic regions that are normally buried within the protein's structure. These exposed hydrophobic regions can interact with similar regions on other protein molecules or cellular components, leading to the accumulation and aggregation of proteins.
How to find Molar Extinction Coefficient?
Protein aggregates can be divided into two categories: amyloid aggregates and amorphous aggregates. Intermolecular -sheets give the highly organized structure of amyloid aggregates, which are perpendicular to the aggregates' central axis. Amorphous aggregates or inclusion bodies exist in addition to amyloid. Although these amorphous aggregates lack long-range organized structures, they do include certain -structures that resemble amyloids. In images produced by an electron microscope, this renders them unstructured.
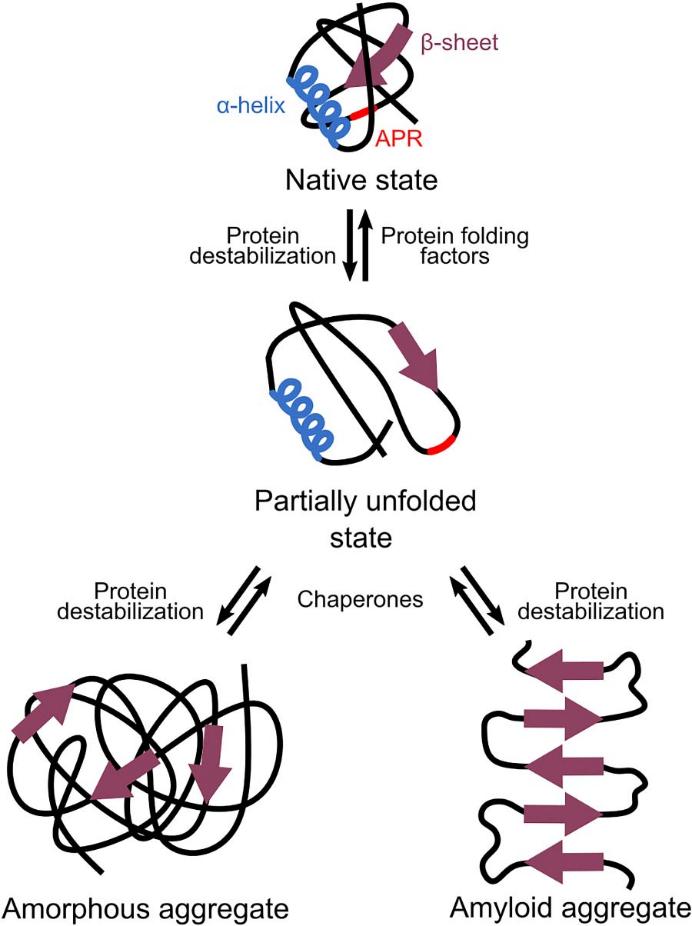 Fig 2. Different types of protein aggregations. (Celien, B., et al.; 2021)
Fig 2. Different types of protein aggregations. (Celien, B., et al.; 2021)
Amorphous or amyloid aggregates are frequently linked to negative consequences, like the loss of function of aggregating proteins. When aggregation-prone areas are present often and cause aggregation across the proteome, this severe loss of function can even be fatal. Amyloid aggregates not only result in loss of function but also have a direct link to cytotoxicity. Rather than the relatively inert mature amyloid itself, this toxicity is typically brought on by soluble oligomers prior to amyloid formation. Possible methods for how these oligomers cause cell death and toxicity include membrane permeabilization and damage. Amorphous aggregates are typically not hazardous, unlike amyloid.
How to Study Protein Aggregation?
1. Biochemical Analytical Techniques
Evaluation of protein aggregation is conducted via biochemical assays utilizing methods such as size exclusion chromatography, native gel electrophoresis, and sedimentation assays. These techniques are crucial in determining the degree of aggregation, identifying the existence of oligomers or fibrils, and understanding the kinetics of aggregation events.
2. Spectroscopic Approaches
Spectroscopic techniques such as fluorescence spectroscopy, circular dichroism (CD) spectroscopy, and Fourier-transform infrared spectroscopy (FTIR) offer valuable tools in the study of protein aggregation. These methods facilitate the monitoring of alternations in protein conformation and secondary structure throughout the aggregation process.
3. Thioflavin T (ThT) Fluorescence Assay
ThT is a specific dye that interacts with amyloid fibrils, often employed to detect and quantify the formation of fibrillar aggregates. The detection measure is based on the fluorescence intensity increase of ThT in the presence of aggregated proteins.
4. Microscopic Methodologies
High-resolution imaging techniques, like electron microscopy (EM) and atomic force microscopy (AFM), deliver visual representation of aggregate structures. Such techniques render crucial data on the morphology, distribution, and size of the aggregates produced during protein aggregation.
5. Experimental Assays
Investigations of the effects of protein aggregation on cellular function and lifespan often utilize cell line or model organism-based assays. These experimental design might require the monitoring of modifications in cellular morphology or viability, or the evaluation of aggregation impact on cellular processes via techniques such as immunofluorescence staining or Western blot analysis.
6. Computational Modeling Approaches
Molecular dynamics simulations along with other computational methods can be harnessed to study the aggregation progression at the atomic level. These simulations deliver invaluable insights into the thermodynamics, kinetics, and structural facets of the protein aggregation phenomenon.
Why Us?
Our Commitment and Future Goals
At Creative Proteomics, we offer cutting-edge services in the area of protein drug characterisation, precisely analyzing protein aggregation using cutting-edge technology. We are a trusted partner in the advancement of scientific knowledge because of our team of subject matter experts and dedication to innovation. Please get in touch with us if you have any specific needs for our protein aggregation analysis services.
References
- Tyedmers, J., et al.; Cellular strategies for controlling protein aggregation. Nature Reviews Molecular Cell Biology. 2010, 11(11), 777–788.
- Celien, B., et al.; Protein Aggregation as a Bacterial Strategy to Survive Antibiotic Treatment. Frontiers in Molecular Biosciences. 2021.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Overview of cellular protein aggregation. (Sani, E., et al.; 2010)
Fig 1. Overview of cellular protein aggregation. (Sani, E., et al.; 2010) Fig 2. Different types of protein aggregations. (Celien, B., et al.; 2021)
Fig 2. Different types of protein aggregations. (Celien, B., et al.; 2021)
