Introduction to Interferon-β (INF-β)
Interferon-β (INF-β) is part of a protein group collectively referred to as interferons (IFNs). Historically, the classification system for IFNs was grounded in the determination of their cellular origins, meaning the primary three divisions were leukocyte-, fibroblast-, and immune-interferons corresponding to their synthesis primarily in leukocytes, fibroblasts, and T-lymphocytes, respectively.
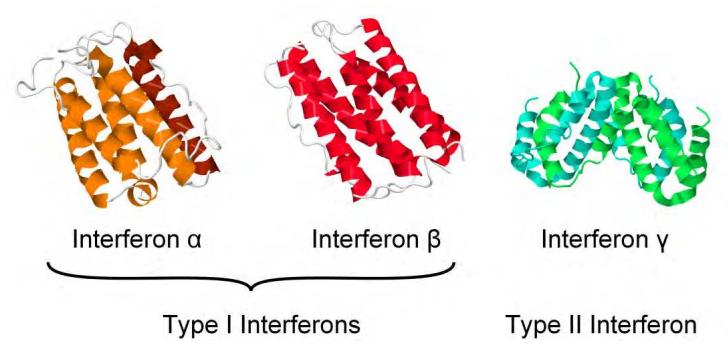 Fig 1. Interferon protein structures. (N., D., & D., B. 2012)
Fig 1. Interferon protein structures. (N., D., & D., B. 2012)
However, as our comprehension of INF structure and functionality has advanced, so has the nomenclature of IFN. Presently, the three prime categories of INF are labelled as INF-α, INF-β, and INF-γ. Between human INF-α and INF-β, there is an approximate 30% similarity at the primary amino acid sequence level. Conversely, INF-γ shares no marked similarity with either.
Structure of INF-β
INF-β is endowed with three cysteine residues that are situated at the 17th, 31st, and 141st positions of the amino acid sequence. It is plausible that these cysteine residues partake in intermolecular disulfide bridging, thereby leading to the creation of inactive dimers and oligomers. Concurrently, a random interaction within each molecule could occur among the three cysteines, leading to the presence of three different molecular species within the cell.
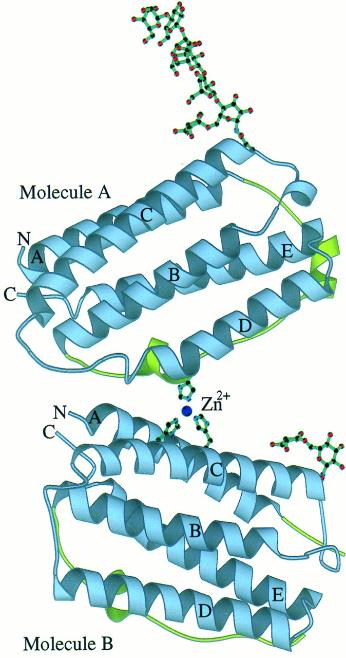 Fig 2. Schematic representation of the crystallographic dimer of huIFN-β. (Karpusas, M., et al. 1997)
Fig 2. Schematic representation of the crystallographic dimer of huIFN-β. (Karpusas, M., et al. 1997)
Each of these possesses one of the three potentially possible intramolecular disulfide bridges. It has been conjectured that only one is likely to mimic the native conformation and hence maintain biological viability. Both these scenarios could collectively contribute to the creation of inactive monomers and oligomers within the cell. If in fact the sulfhydryls were accountable for the diminished specific activity of the INF-β protein, then the exclusion of one of the cysteines could make way for the sole unique intramolecular disulfide bridge formation. Thereby, subsequently limiting the formation of free-sulfhydryl groups that can facilitate the generation of dimers. or oligomers.
Stability of INF-β
The stability of INF-β has been found to be associated with its formulation parameters. A non-carrier protein solution formulation of INF-β, with a per milliliter composition of 1.2 mg INF-β, 10 mg of SDS in a 50 mM sodium acetate base and 2 mM EDTA, and a pH of 5.5, was used in the experiments. We subjected the collected data to an Arrhenius fit. The resultant t90 value, indicative of the time taken to achieve 90% purity of Interferon, derived from the SDS-PAGE and RP-HPLC data, was predicted to be around 7 years at 5°C, within a range of 2–8°C. Moreover, the rate of INF-β degradation was observed to have an activation energy of 24 kcal/mole.
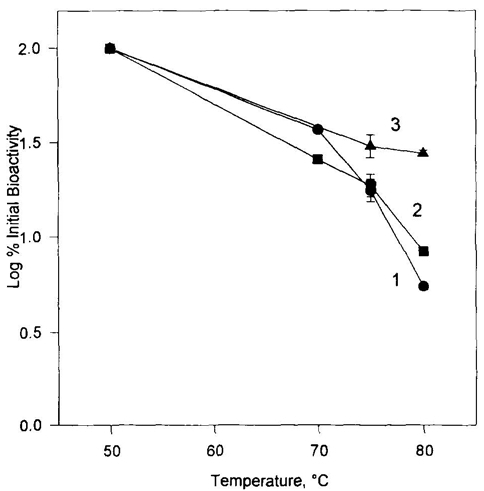 Fig 3. Potency stability of INF-β at 5°C. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Potency stability of INF-β at 5°C. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
What Can We Offer?
By leveraging leading-edge techniques and cutting-edge technologies, Creative Proteomics offers a comprehensive suite of protein drug characterization services. Our highly skilled team of researchers, with their in-depth knowledge, guarantee precise and reliable analyses, thereby enhancing the complexity of understanding the characteristics, properties, and functions of protein drugs. For more detailed exploration of our services, you are more than welcome to contact us.
References
- N., D., & D., B. Interferon and Apoptosis in Systemic Lupus Erythematosus. Systemic Lupus Erythematosus. 2012.
- Karpusas, M., et al. The crystal structure of human interferon at 2.2-A resolution. Proceedings of the National Academy of Sciences. 1997, 94(22), 11813–11818.
- Pearlman, Rodney, and Y. John Wang, eds. Formulation, characterization, and stability of protein drugs. Vol. 9. Springer Science & Business Media. 1996.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. Interferon protein structures. (N., D., & D., B. 2012)
Fig 1. Interferon protein structures. (N., D., & D., B. 2012) Fig 2. Schematic representation of the crystallographic dimer of huIFN-β. (Karpusas, M., et al. 1997)
Fig 2. Schematic representation of the crystallographic dimer of huIFN-β. (Karpusas, M., et al. 1997) Fig 3. Potency stability of INF-β at 5°C. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Potency stability of INF-β at 5°C. (Pearlman, Rodney, and Y. John Wang, eds. 1996)