Introduction to Recombinant Human GM-CSF
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a type of cytokine growth factor that plays a crucial role in promoting the growth and function of white blood cells. GM-CSF specifically acts on partially committed progenitor cells to stimulate their division and differentiation along the pathways leading to the development of granulocytes and macrophages. Additionally, GM-CSF elicits a cellular response in mature end-stage cells of the granulocyte, monocyte, macrophage, and eosinophil lineages.
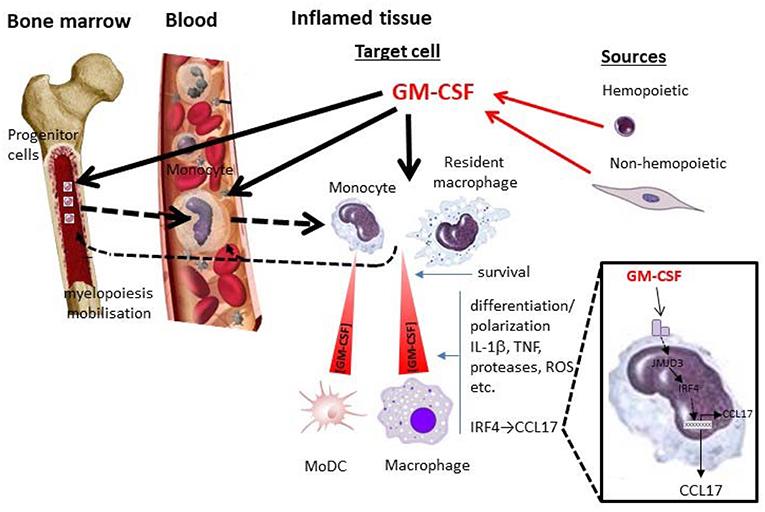 Fig 1. GM-CSF and monocytes/macrophages in inflammation. (Hamilton, John A. 2019)
Fig 1. GM-CSF and monocytes/macrophages in inflammation. (Hamilton, John A. 2019)
The biological activity of GM-CSF is mediated through its interaction with cell-surface receptors. These receptors exist in both low- and high-affinity states. The low-affinity receptor, referred to as the α-subunit, is specific to GM-CSF. On the other hand, the high-affinity receptor is a heterodimer composed of both the α-subunit and a β-subunit that is shared with the receptors for other cytokines such as IL-3 and IL-5. It is important to note that the formation of the high-affinity receptor only occurs when both the α- and β-subunits are bound to GM-CSF.
Structural Studies of GM-CSF
GM-CSF is an acidic glycoprotein that belongs to the class of hematopoietic growth factors. In order to better understand the structure of non-glycosylated GM-CSF, studies were carried out using X-ray crystallography techniques. The results showed that GM-CSF consists of a bundle of four α-helices arranged in a left-handed antiparallel arrangement, and a unique folded structure, in which a double-stranded antiparallel β-sheet is fused with four α-helices, was found in GM-CSF.
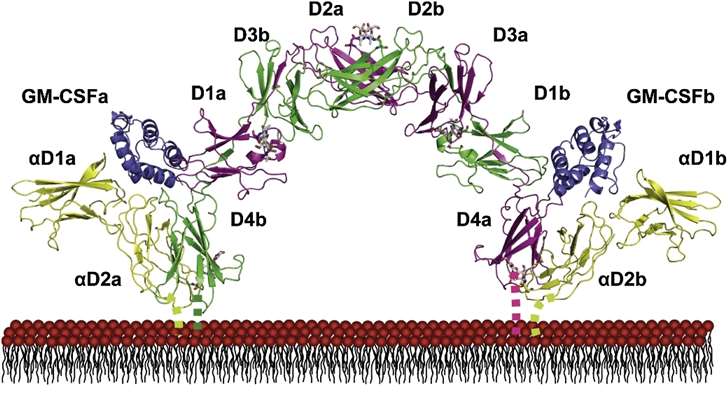 Fig 2. Structure of the GM-CSF Receptor Ternary Complex. (Hansen, Guido, et al. 2008)
Fig 2. Structure of the GM-CSF Receptor Ternary Complex. (Hansen, Guido, et al. 2008)
Protein Integrity by SDS-PAGE Silver Stain
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was utilized to assess GM-CSF protein integrity. To accomplish this, a 10%-20% linear gradient gel was used to discriminate protein species based on apparent molecular weight. Subsequently, silver staining was utilized for detection.
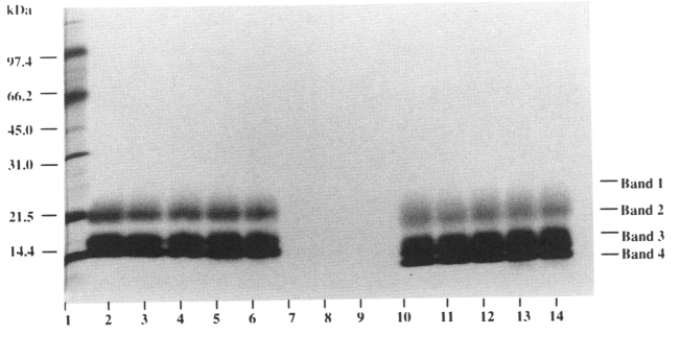 Fig 3. Stability evaluation by SDS-PAGE. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Stability evaluation by SDS-PAGE. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
This method proved to be effective in identifying potential protein fragmentation and oligomerization. Notably, recombinant human GM-CSF can be divided into three major glycosylated components, bands 4, 3, and 2, which have apparent molecular weights ranging from 14 to 21 kDa. In addition, there is a highly glycosylated component, referred to as band 1, which usually exhibits a broad band above 25 kDa.
Protein Aggregation by SE-HPLC
Size exclusion high performance liquid chromatography (SE-HPLC) was utilized to determine protein aggregation in GM-CSF. The monomeric and aggregated proteins were separated based on their apparent molecular size. The separation process was performed using a Bio-Sil 125 column equipped with an isocratic aqueous solvent of 100 mM sodium phosphate and 150 mM sodium chloride at pH 7.2. To ensure accurate detection and monitoring, elution was continuously observed through absorbance readings at 220 nm. By applying this method, recombinant human GM-CSF was divided into doubled monomeric and aggregated components.
What Can We Offer?
Leveraging sophisticated techniques and cutting-edge technologies, Creative Proteomics offers a comprehensive array of protein drug characterization services. Our team, comprising skilled scientists with deep-rooted expertise, assures accurate and reliable analyses, fostering a thorough comprehension of the characteristics, properties, and functional mechanisms of protein drugs. For further inquiries or exploration of our services, feel free to initiate a conversation with us.
References
- Hamilton, John A. GM-CSF-dependent inflammatory pathways. Frontiers in immunology. 2019.10: 2055.
- Hansen, Guido, et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008. 134.3: 496-507.
- Pearlman, Rodney, and Y. John Wang, eds. Formulation, characterization, and stability of protein drugs. Vol. 9. Springer Science & Business Media. 1996.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. GM-CSF and monocytes/macrophages in inflammation. (Hamilton, John A. 2019)
Fig 1. GM-CSF and monocytes/macrophages in inflammation. (Hamilton, John A. 2019) Fig 2. Structure of the GM-CSF Receptor Ternary Complex. (Hansen, Guido, et al. 2008)
Fig 2. Structure of the GM-CSF Receptor Ternary Complex. (Hansen, Guido, et al. 2008) Fig 3. Stability evaluation by SDS-PAGE. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Stability evaluation by SDS-PAGE. (Pearlman, Rodney, and Y. John Wang, eds. 1996)