ICH Q6B states "For the special case of adventitious viral or mycoplasma contamination, the concept of action limits is not applicable, and the strategies proposed in ICH Harmonised Tripartite Guidelines should be considered."
With an experienced team of scientists utilizing advanced techniques, Creative Proteomics ensures accurate, reliable results. This mycoplasma testing service can help labs, researchers, and organizations maintain the quality of their biological materials, contributing to credible and reproducible research results.
Mycoplasma Testing
Mycoplasma Testing is a method used to determine if cell cultures used in biological and medical research and production are contaminated with mycoplasma bacteria. Mycoplasma are a type of bacteria that lack a cell wall and are small in size, which makes them particularly difficult to detect and remove. This contamination can adversely affect cellular functions including gene expression and metabolism, and can introduce significant issues in the reproducibility and reliability of research results.
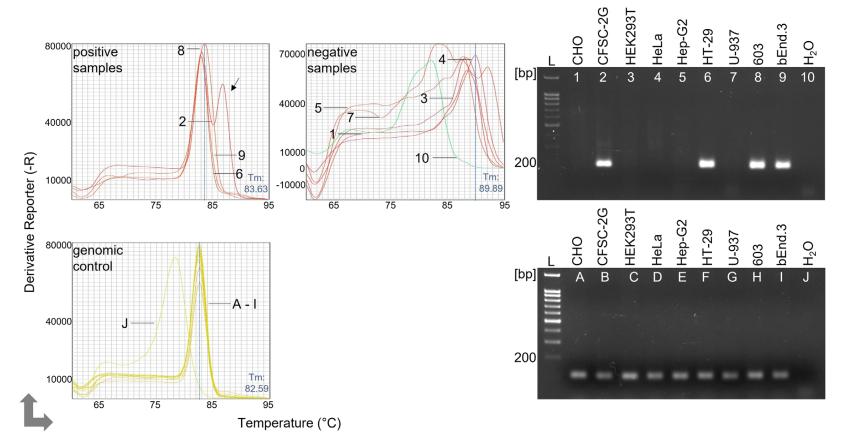 Fig 1. Detection of mycoplasma infection in different cell lines using qPCR. (Siegl, Dominik, et al. 2023)
Fig 1. Detection of mycoplasma infection in different cell lines using qPCR. (Siegl, Dominik, et al. 2023)
Regulatory Compliance
Mandatory mycoplasma testing is stipulated in various global regulations, compendia and guidelines, and there are several test methods to fulfill these requirements. Among these methods, the traditional and indicator cell culture techniques are somewhat inconsistent and can vary depending on the jurisdiction.
These tests are developed in compliance with the specific regulatory guidelines noted in the United States Pharmacopeia (USP) General Chapter <63>, European Pharmacopoeia (EP) General Chapter 2.6.7, and Japanese Pharmacopoeia (JP).
Mycoplasma Testing Methods
1. Endpoint PCR detection methods (regular PCR, touchdown PCR, reverse transcription-PCR)
Drawing parallels to DNA, one can also identify the presence of mycoplasma RNA within biologics through the employment of endpoint RT-PCR (reverse transcriptase-PCR); a method commonly used in molecular biology. Moreover, there are numerous alternative RNA amplification methodologies that can be used to augment RNA targets. These extend beyond conventional RT-PCR and include nucleic acid sequence-based amplification (NASBA) as well as transcription-mediated amplification (TMA).
2. Nucleic acid testing (NAT) methods
The main benefit of Nucleic Acid Testing (NAT) lies in its capacity to offer swift, sequence-specific, and significantly sensitive identification of target DNA or RNA within multi-component biological specimens. A range of NAT assays modifications have also been configured for the detection and determination of mycoplasma agents across various types of clinical tokens and biological materials. This broad scope extends to continuous cell cultures, autologous cultured cells, raw materials, bioreactor harvests, and other diverse examples.
3. Microarray based methods for detection and identification

Microarray technology enables both the detection and species-level identification of mycoplasma contaminants. This can be attributed to the function of microarray oligonucleotide species-specific probes. These probes, tethered to or synthesized directly on a solid microarray platform, can uniquely identify targeted sequences present within mycoplasma genomes.
4. Massively parallel sequencing (MPS)
Multiplex Sequencing (MPS) serves as an influential parallel approach for high-throughput sequencing procedures. This provides a considerable amenity for the concurrent detection and identification of multiple unforeseen agents in multifaceted biological samples, irrespective of preliminary cognizance regarding the characteristics of these agents. Empirical evidence substantiates that the conjunction of MPS and random priming polymerase chain reaction (PCR) possesses the capability to unearth adventitious agents present within cellular substrates or viral seed stocks.
Service Process
Want to Learn More?
As a leader in the biotechnology sector, Creative Proteomics offers an exceptional mycoplasma testing service. Equipped with advanced scientific instruments and a cadre of expert scientists, we are dedicated to providing accurate and swift test results. Our comprehensive mycoplasma testing solutions grant assurance to individuals, academic establishments and industrial organizations. For more information regarding our services, do not hesitate to reach out to us.
Reference
- Siegl, Dominik, et al. A PCR protocol to establish standards for routine mycoplasma testing that by design detects over ninety percent of all known mycoplasma species." Iscience. 2023, 26.5.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Detection of mycoplasma infection in different cell lines using qPCR. (Siegl, Dominik, et al. 2023)
Fig 1. Detection of mycoplasma infection in different cell lines using qPCR. (Siegl, Dominik, et al. 2023)




