ICH Q6B states "Characterisation of a biotechnological or biological product (which includes the determination of physicochemical properties, biological activity, immunochemical properties, purity and impurities) by appropriate techniques is necessary to allow relevant specifications to be established."
Identifying and characterizing protein impurities is a substantial component of affirming the quality, safety, and efficacy of protein-centric products. To tackle this complex area, Creative Proteomics employs cutting-edge methodologies and instrumental techniques while leveraging the expertise of a very proficient team of scientists. This allows them to identify, analyze, and quantify protein impurities with a high degree of accuracy and precision.
What is Protein Impurities?
Protein impurities denote undesirable or non-intentional proteins interspersed within a given protein sample. The advent of these impurities can be attributed to diverse steps in the protein extraction and purification procedures or due to inadvertent contamination. These contaminants have the potential to compromise the precision of scientific research and efficacy of protein-derived therapeutics, hence posing a significant risk to safety parameters. As such, the identification and elimination of these impurities to guarantee protein sample purity is paramount in both the academic research sphere as well as the pharmaceutical industry.
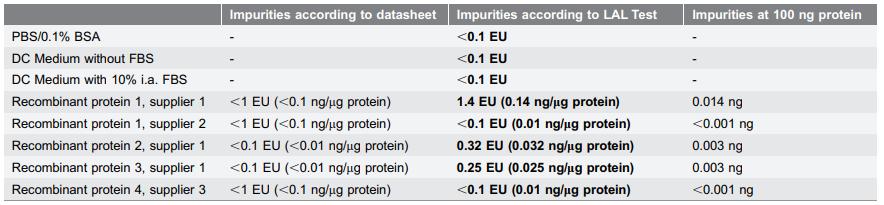 Fig 1. Measured impurities in protein preparations from different suppliers evaluated by LAL tests. (Schwarz, H., et al.; 2014)
Fig 1. Measured impurities in protein preparations from different suppliers evaluated by LAL tests. (Schwarz, H., et al.; 2014)
Why do Protein Impurities Analysis?
The application of protein impurity analysis services is multi-fold. Here are several key reasons:
Patient Safety: P Protein-based drugs are made through processes like gene cloning and protein purification, during which unwanted changes like mutations can occur, creating impurities. These impurities can potentially harm patients and affect the medicine's effectiveness. Therefore, by conducting comprehensive protein impurity analyses, one can ensure that the levels of impurities present in the drug formulation conform to the established regulatory guidelines, thereby guaranteeing optimization of patient safety.
Quality Control: Besides safety assessments, the premise of quality control plays a crucial role in fabricating protein-based therapeutics. These assays accurately determine the quality and purity of the biologic bearing product, thus ensuring its safety and intended therapeutic action.
Improved Processing Techniques: An in-depth inquiry into the nature and extent of protein impurities offers valuable insights into the limitations and problematic domains encountered during the production and processing of protein-based drugs. Armed with this knowledge, one can refine processing techniques to increase both the quality of product yield and the overall therapeutic efficacy of the drug.
Importance of Protein Impurities Analysis
Immunogenic Implications

Proteinaceous impurities pose a significant risk owing to their potential to trigger an undesired immune response, a phenomenon termed immunogenicity. This occurs when the immune system perceives these impurities as foreign antigens, initiating an immune response ranging from transient mild reactions to severe life-threatening events such as anaphylaxis. In certain circumstances, the immune system may generate antibodies to the biologic treatment, which could abrogate its therapeutic efficacy.
Variations in Therapeutic Efficacy
Protein malcontaminations could interact with a biologic medication, impacting its structure and function, ultimately attenuating its potency and efficacy. This results in sub-optimal therapeutic outcomes. Additionally, batch-to-batch variations in protein impurity levels can induce inconsistency in the drug's performance.
Safety Implications

Protein contaminations could potentiate drug toxicity, thereby endangering patient safety. Some impurities may exert direct cytotoxic effects on specific cell types or interfere with fundamental biological processes.
Regulatory Compliance
Regulatory authorities maintain strict standards for the purity and quality of biologic drugs. The presence of protein impurities may result in non-compliance with these standards, potentially leading to product recalls, penalties, and reputational harm to the manufacturer.
Drug Stability

Protein contaminants may adversely impinge on the stability of biologic drugs. For instance, these impurities can foster the undesirable aggregation of drug molecules, causing a loss of drug activity and possible immunogenicity.
Drug Delivery Impact

In certain cases, protein contaminants could affect the dosage and delivery of biologic treatments. Such impurities could influence the solubility or viscosity of the drug, thereby affecting its administration and possibly resulting in unsuccessful treatment.
Manufacturing Challenges
The existence of protein contaminants generally warrants extra purification steps, increasing production time and costs. This escalation in production expenses could in turn impact the final cost and market availability of the drug.
What can We Provide for Protein Impurities Analysis?
As an esteemed provider in the field, Creative Proteomics provides an extensive array of services related to Host Cell Protein (HCP) Analysis, fulfilling both the research and industry-related requirements. Our highly advanced technological setup, backed by a team of seasoned scientists, facilitates meticulous research and characterization of HCP entities in diverse biological samples. Creative Proteomics leverages customized HCP analysis methodologies to provide their clientele with vital data related to the quality, safety, and functionality of their products. This is carried out while strictly adhering to regulatory standards, hence providing the foundation for smooth progress in drug development and subsequent commercialization.
Creative Proteomics, a global, preeminent entity in the bioscientific research realm, proudly introduces an advanced, holistic residual host cell DNA (HCD) analysis service. This service is meticulously engineered to conduct exhaustive inspection and evaluation of residual host cell DNA that may persist in biopharmaceuticals following manufacturing undertakings. Deploying sophisticated scientific techniques and a cadre of highly experienced academicians, Creative Proteomics guarantees an elevated level of precision and dependability in their analysis offering. This ensures that medical firms and pharmaceutical businesses adhere to regulatory norms, maintain product safety, and actualize substantive quality augmentation in their products.
As a leading figure in the domains of proteomic and genomic analysis, Creative Proteomics provides a specialized service termed residual protein A analysis. This particular approach aims to detect and quantify residual protein A, a bacterial protein frequently employed in the production of therapeutic antibodies. Residual protein A can present issues if not adequately removed from the final product, with potential triggers of adverse immune responses in patients. Hence, it is crucial to precisely characterize and limit the presence of residual protein A during biotherapeutic production processes. Creative Proteomics leverages state-of-the-art technologies to execute this crucial service, thus delivering significant data to assist clients in verifying the safety and effectiveness of their biotherapeutic developments.
At Creative Proteomics, we are pioneers in advanced scientific research and provide an invaluable service focusing on the analysis of process-related impurities and residuals. This service is especially pertinent in industries such as pharmaceuticals, food and beverage, and chemical products, and plays a crucial role in guaranteeing the safety and quality of consumer products. We employ cutting-edge techniques to identify, quantify, and track impurities and residuals emerging during manufacturing and processing. This range of impurities may encompass raw materials, catalysts, solvents, intermediates, byproducts, and even decomposition products. By offering a complete analysis, we provide insightful knowledge of your systems. This expertise can facilitate troubleshooting procedures, enhance efficiency, and ensure adherence to industry regulations and standards.
Sample Requirement
| Sample Type |
Protein |
Cell |
Animal tissue |
Plant Tissue |
Blood (EDTA added) |
Serum |
Urine |
Microbes |
| Quantity |
100 ug |
2X107 cells |
1g |
200 mg |
1mL |
0.2-0.5 mL |
2 mL |
Dry weighed: 200 mg |
Our Advantages
Want to Learn More?
Creative Proteomics, an innovator in proteomics services, offers support in identifying protein contaminants, critical in areas like pharmaceutical development and protein-focused research. Our experts use advanced methods to accurately identify the traits of these contaminants, refining purification processes and ensuring the final product meets regulatory standards. We invite you to reach out to us.
Reference
- Schwarz, H., et al.; Residual Endotoxin Contaminations in Recombinant Proteins Are Sufficient to Activate Human CD1c+ Dendritic Cells. PLoS ONE.2014, 9(12), e113840.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Measured impurities in protein preparations from different suppliers evaluated by LAL tests. (Schwarz, H., et al.; 2014)
Fig 1. Measured impurities in protein preparations from different suppliers evaluated by LAL tests. (Schwarz, H., et al.; 2014)







