To comprehend the structure and function of proteins, uncover biological pathways, and develop new medications, Creative Proteomics offers comprehensive secondary structure analysis of proteins that enable custemers meet the biotechnology/biologics acceptance criteria of the ICH Q6B guidelines.
Why do Protein Secondary Structure Analysis?
Protein secondary structure refers to the local folding patterns or arrangements of the protein's backbone atoms (mainly the alpha carbon) in a polypeptide chain. It describes the regular repetitive patterns that form between amino acids in close proximity to each other.
The two most common types of protein secondary structures are the alpha helix and the beta sheet.
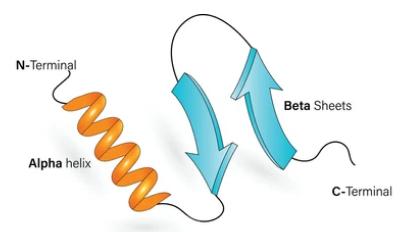 Fig 1. Protein secondary structure.
Fig 1. Protein secondary structure.
1. Alpha Helix: In an alpha helix, the polypeptide chain spirals in a right-handed manner, forming a cylindrical or helical structure. The backbone atoms are held together by hydrogen bonding between the carbonyl oxygen of one amino acid and the amide hydrogen of a nearby amino acid, typically located four amino acids ahead in the sequence. This hydrogen bonding pattern gives the alpha helix its stability.
2. Beta Sheet: In a beta sheet, the polypeptide chain folds back and forth forming a pleated sheet-like structure. The backbone atoms are held in place by hydrogen bonding between the carbonyl oxygen of one strand and the amide hydrogen of a nearby strand. Beta sheets can be either parallel, where the neighboring strands run in the same direction, or antiparallel, where the neighboring strands run in opposite directions.
The secondary structure of a protein is primarily determined by the interactions between the amino acid residues, such as hydrogen bonding, van der Waals forces, and hydrophobic interactions. It is important for protein stability, function, and overall three-dimensional structure.
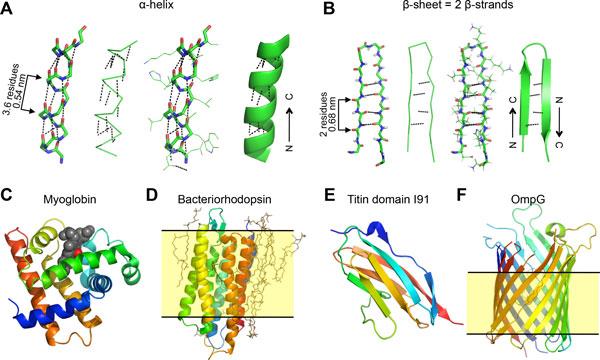 Fig 2. Secondary structure of proteins. (Rico, F., et al.; 2013)
Fig 2. Secondary structure of proteins. (Rico, F., et al.; 2013)
Protein Secondary Structure Analysis Methodology
X-ray crystallography functions as a tool for rigorous high-resolution elucidation of protein structures, providing nuanced insights into the accurate disposition of secondary structure components.
Circular Dichroism spectroscopy quantifies the differential absorption of left and right circularly polarized light, thus yielding valuable data about the overall secondary structure composition of a protein.
Nuclear Magnetic Resonance spectroscopy is instrumental in capturing the dynamics of protein structures in solution, allowing the identification and analysis of fleeting secondary structure phenomena in proteins.
Challenges in Protein Secondary Structure Analysis
Ambiguity: Experimental methods like X-ray crystallography or NMR spectroscopy can provide high-resolution structural information, but they are time-consuming and costly. On the other hand, computational methods rely on predictive algorithms that may have limitations in accurately predicting the secondary structure.
Diversity: Proteins can adopt various secondary structure elements such as α-helices, β-sheets, turns, and coils. The prediction of these elements can be difficult due to the diversity and flexibility of protein structures.
Sequence-dependent effects: The secondary structure of a protein can be influenced by its amino acid sequence. Certain amino acid residues have a propensity to form specific secondary structures, but this propensity can be modulated by neighboring residues or environmental factors, making accurate prediction challenging.
Disordered regions: Proteins can also contain regions that lack a well-defined secondary structure, known as intrinsically disordered regions. These regions can be challenging to analyze as they do not conform to the regular secondary structure elements.
What We Provide
Predict the three-dimensional structure of proteins: Since the tertiary configuration of proteins is vitally derived from its secondary construction, it is viable to prognosticate the tertiary framework by conducting thorough scrutiny of their secondary organization. This pivotal investigation can provide significant insights into comprehending the structural and functional aspects of proteins more profoundly.
Examine the connection between protein structure and function: The structure and correlative function of proteins showcase an intrinsic bond. To explore this bond, it is essential to unravel the impact of amino acids in differing structural iterations on the protein's structure and function. This can be realized by conducting an exhaustive analysis of a protein’s secondary structure.
Recognize the flexibility of proteins: The secondary framework of proteins exhibits the potential to mutate according to environmental fluctuations. A thorough analysis of these secondary structures elucidates the dynamic nature of proteins, further revealing their vital role and functional modus operandi within organisms.
Design innovative proteins: An extensive study of the secondary structure of proteins empowers us to comprehend the consequential impact of amino acids of diverse structures on the form and operation of proteins. Armed with this knowledge, it becomes feasible to engineer unique proteins bespoke to specific biological requirements or even to innovate new medicinal compounds.
Service Workflow
Our Commitment and Future Goals
In order to maintain its position as a leader in protein secondary structure analysis, Creative Proteomics is committed to constantly updating and improving our analytical methods and services. To offer even more thorough insights into protein structure, we intend to broaden the scope of the procedures and tools we use. We want to establish ourselves as a reliable partner for researchers and biopharmaceutical firms in their quest to comprehend and characterize protein secondary structure by fusing cutting-edge technology, professional analysis, and a client-centric approach.
If you are interested in our services or have any other questions, please contact us, we would appreciate hearing from you and look forward to working with you.
Reference
- Rico, F., et al.; Mechanics of proteins with a focus on atomic force microscopy. Journal of Nanobiotechnology. 2013, 11(Suppl 1), S3.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Protein secondary structure.
Fig 1. Protein secondary structure. Fig 2. Secondary structure of proteins. (Rico, F., et al.; 2013)
Fig 2. Secondary structure of proteins. (Rico, F., et al.; 2013)