At Creative Proteomics, we specialize in the comprehensive analysis of residual protein A, catering specifically to the needs of organizations within the biopharmaceutical sector that focus on monoclonal antibody drugs. We offer our expertise to support businesses in achieving superior standards in biopharmaceutical quality control, ensuring compliance with the established regulatory ICH Q6B guidelines. As professionals with backgrounds in biology-related disciplines, we champion meticulous services and advanced solutions in the field of biopharmaceuticals.
Why do Residual Protein A Analysis?
Residual Protein A is a protein component that can occasionally remain in small trace amounts following the process of pharmaceutical manufacturing, more specifically in the development of monoclonal antibodies. Monoclonal antibodies are a key type of bioengineered protein tailored to imitate the immune system's action of neutralizing harmful pathogens such as bacteria or viruses.
Within the manufacturing process, Protein A, a specific type of bacterial protein, is often utilized to purify these monoclonal antibodies due to its ability to bind to antibodies, thereby facilitating their isolation. Despite the important role of Protein A in the purification process, it is imperative to reduce its quantity in the final product as low as possible. The main concern lies in its potential to provoke an immune response in patients receiving treatments which contain it. This response could give rise to undesirable health complications, varying from minor reactions to severe conditions.
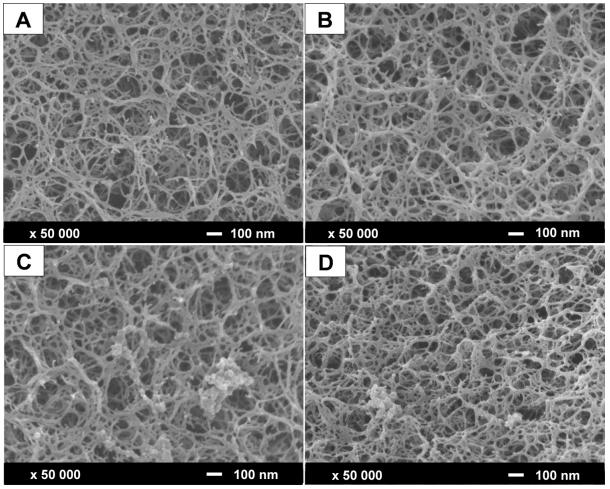 Fig 1. Scanning electron microscopy images of the protein A resin surface. (Lintern, K., et al.; 2016)
Fig 1. Scanning electron microscopy images of the protein A resin surface. (Lintern, K., et al.; 2016)
The strict monitoring, regulation, and control of residual Protein A levels in the pharmaceutical manufacturing process is crucial for maintaining quality standards and patients' safety. This is achieved through quality control measures, testing protocols, and purification techniques. The presence of Protein A is not insignificant, as it optimizes the effectiveness of monoclonal antibodies. Managing associated risks and maximizing benefits is vital for ensuring patients' wellbeing and health in clinical settings.
What Can We Offer for Residual Protein A Analysis?
Enzyme-Linked Immunosorbent Assay (ELISA)
The Enzyme-Linked Immunosorbent Assay (ELISA) serves as a prevalent technique in detecting residual protein A. This process effectively utilizes specific antibodies designed for the purpose of binding to protein A. Subsequent detection is accomplished via an enzyme-driven reaction, manifesting in either colorimetric or fluorescent form.
Western Blotting
This methodology encompasses the segmentation of proteins via gel electrophoresis, followed by their transference to a membranous structure. The membrane is subjected to probing with specific antibodies designed for protein A, consequently facilitating the visible identification of the said protein through a sophisticated detection system.
Mass Spectrometry (MS)
MS is a robust analytic procedure that enables the recognition and characterization of distinct protein molecules. Utilized meticulously, it presents the capability of identifying and quantifying residual Protein A via the measurement of the mass-to-charge ratio of protein fragments, which can be invaluable when delving into detailed organismal and sub-organismal biological studies.
Capillary Electrophoresis (CE)

CE is a distinguished and sophisticated chromatographic technique deployed commonly to distinguish proteins as per their respective charge-to-size ratio. Under this method, Protein A, to illustrate, can be meticulously isolated from the sample matrix, subsequent to which it can be accurately detected and enumerated through ultraviolet absorption or the implementation of fluorescence detection.
Polymerase Chain Reaction (PCR)
qPCR techniques are employed for the identification and analysis of the DNA sequence specific to Protein A genes. The amplification of this DNA segment and subsequent analysis allows the verification of residual presence of Protein A.
Workflow for Residual Protein A Analysis
- Sample Preparation: Prior to any analysis, the sample must undergo appropriate preparation methods, such as dilution or protein extraction. Certain techniques including filtration, centrifugation or chromatography may be necessitated, depending on the specific nature of the sample.
- Antibody Bind: To secure residual Protein A, a distinctive antibody is employed. Various commercial antibodies against Protein A can be immobilized on solid substrates such as microplates or magnetic beads for this purpose.
- Washing Process: To ensure the elimination of contaminants or non-specific bindings, the captured sample is subjected to multiple washing steps using buffer solutions. This step assures that only Protein A remains affixed to the immobilized antibody.
- Detection Method: A uniquely bound detection reagent, typically an antibody or enzyme conjugate labeled with a detectable marker, is employed to Reciprocal Protein A. Persistently, the second form of antibody bears specificity for Protein A and carries a recognizable marker like fluorescent dye or an enzyme that yields a colorimetric or luminescent signal.
- Signal Induction: To enable signal generation, an incubation period is essential to allow the binding between the sample and detection reagent. This incubation period is time-specific and is crucial for measurable signal creation.
- Signal Quantification: Different techniques are applied to measure the signal based on the utilized detection reagent. A fluorometer will be suitable to use, for instance, when a fluorescent dye labels the secondary antibody. Conversely, an enzyme-based reaction requires the use of a spectrophotometer.
- Data Interpretation: Obtained signals or measurements are thoroughly analyzed to quantify the remaining Protein A level in the sample. The standard curve generated by known Protein A concentrations is crucial for the comparative analysis of the obtained sample signal. This curve assists in determining the precise residual Protein A quantity.
Our Advantages
- Deep Expertise: Our expert team has a deep understanding of analytical methods required for precise protein A quantification, thanks to their proficiency in residual protein A evaluation.
- Advanced Technology: We have a unique advantage through access to advanced technology for protein A evaluation. Our labs are equipped with top-tier machinery allowing for highly sensitive and precise analysis.
- Expedited Turnaround: We promise to meet agreed deadlines with high-quality results in the pharmaceutical sphere to keep your business and project on track.
Want to Learn More?
Leading authority, Creative Proteomics, provides reliable residual protein A analysis services in proteomics research, using advanced technology. This evaluation ensures the safety and quality of therapeutic proteins. We encourage you to reach out to us for any queries.
Reference
- Lintern, K., et al.; Residual on column host cell protein analysis during lifetime studies of protein A chromatography. Journal of Chromatography A. 2016, 1461, 70–77.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Scanning electron microscopy images of the protein A resin surface. (Lintern, K., et al.; 2016)
Fig 1. Scanning electron microscopy images of the protein A resin surface. (Lintern, K., et al.; 2016)




