Creative Proteomics excels in offering a diverse array of services encompassing proteomics, genomics, and bioinformatics. We have formulated a highly specialized service focused on the meticulous examination of bacterial endotoxins. It is paramount to emphasize that Creative Proteomics prides itself on delivering services that strictly adhere to the guidelines outlined in the ICH Q6B directive, thus instilling confidence and trust in our extensive clientele.
Overview
Endotoxins produced by bacteria, notably found in the outer membrane of Gram-negative bacteria cells, represent a significant area of concern within both medical and pharmaceutical contexts. These toxic substances are primarily released during bacterial cell disruption or decomposition processes. Lipopolysaccharide (LPS) is the most frequently encountered type of these bacterial endotoxins.
The potential health implications of these endotoxins are significant, with the scope to invoke a myriad of adverse effects in humans. They can trigger a range of toxic impacts, notable among which include alterations in white blood cell count, the induction of fever, and the potential provocation of shock. Given these considerations, the continued study and monitoring of bacterial endotoxins are of extreme importance for the safety of medical practice and pharmaceutical production.
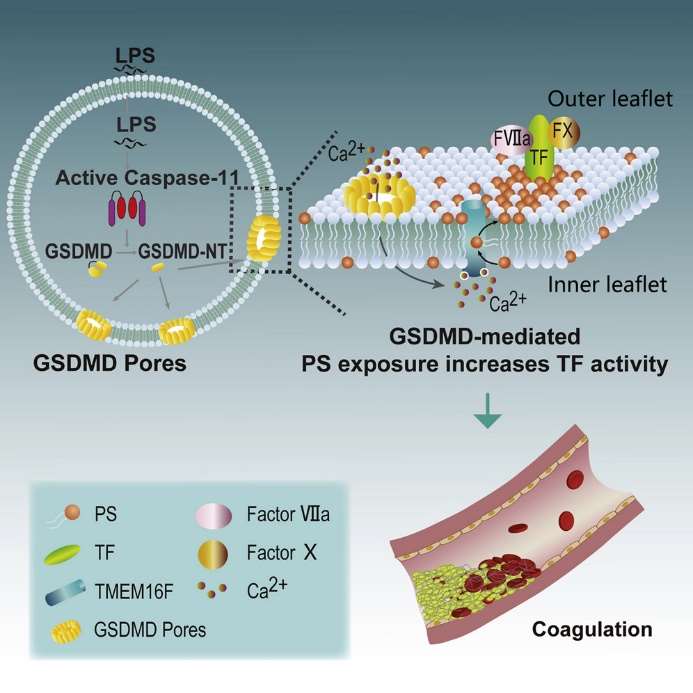 Fig 1. Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. (Yang, X., et al.; 2019)
Fig 1. Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. (Yang, X., et al.; 2019)
Sources of Bacterial Endotoxins
- Gram-negative bacteria: Predominantly found in Gram-negative bacterial species such as Escherichia coli, Salmonella, Klebsiella, Pseudomonas, and Haemophilus, bacterial endotoxins hold significant presence.
- Lipopolysaccharides (LPS): Lipopolysaccharide (LPS) is a multifaceted molecular structure comprised of lipids and saccharides, an example being Lipid A. This molecule serves as an integral component of the bacterial cell wall, establishing the outermost layer which operates as a protective interface between the bacterium and its surrounding environment.
- Cell lysis: When Gram-negative bacterial infections occur, endotoxins are released into the immediate environment as a result of the rupture of bacterial cell membranes. This phenomenon often transpires when such bacteria are annihilated either by the host's immune response or following an antibiotic intervention.
- Contaminated products: Endotoxins produced by bacteria have the potential to contaminate a wide array of products such as pharmaceuticals, medical machinery, and intravenous fluids within the production process. Incidences of contamination may often be attributed to insufficient quality management, non-comprehensive sterilization procedures, or mishandling during the manufacturing phase.
Endotoxin Hypersensitivity
Endotoxin hypersensitivity, alternately referred to as endotoxin tolerance or endotoxin hyporesponsiveness, is delineated by an amplified immune response to endotoxins, a specific category of bacterial exotoxins. These endotoxins predominantly reside in the external membrane of Gram-negative bacteria and are susceptible to liberation into the circulatory system during an infectious episode or specific medical interventions.
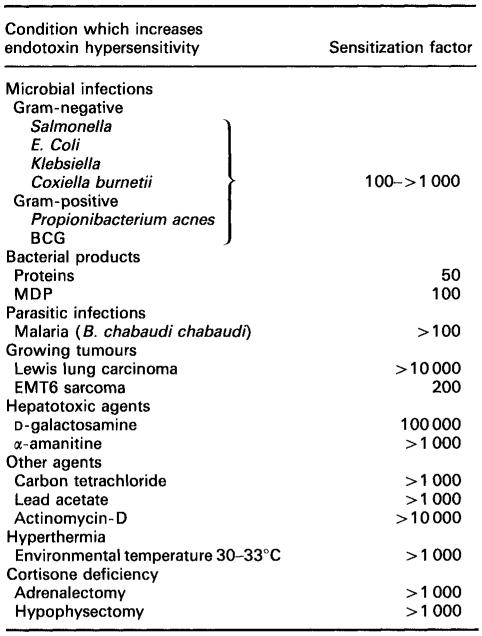 Fig 2. Risk analysis for HCP control during bioprocess development. (Galanos, C., & Freudenberg, M. A.; 1993)
Fig 2. Risk analysis for HCP control during bioprocess development. (Galanos, C., & Freudenberg, M. A.; 1993)
Bacterial Endotoxins Testing
1. Limulus Amebocyte Lysate (LAL)
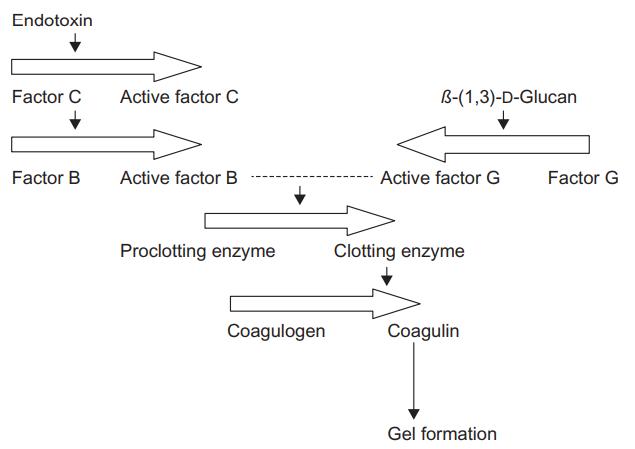 Fig 3. LAL-clotting cascade. (Sandle, T.; 2016)
Fig 3. LAL-clotting cascade. (Sandle, T.; 2016)
The widely employed Limulus Amebocyte Lysate (LAL) assay is a benchmark procedure for the detection and quantification of bacterial endotoxins within any particular specimen. This diagnostic method is resultant of the remarkable defensive mechanisms of the horseshoe crab (Limulus polyphemus), more specifically the blood latent lysate extracted from its amebocytes. Conclusively, due to the extreme sensitivity of this assay, it is crucial to conduct the experiment under rigorous aseptic conditions to circumvent potentially invalid positive results. Therefore, all apparatus, reagents, test surfaces, and glassware should be scrupulously cleaned and free of any interfering substances.
2. Rabbit Pyrogen Test (RPT)
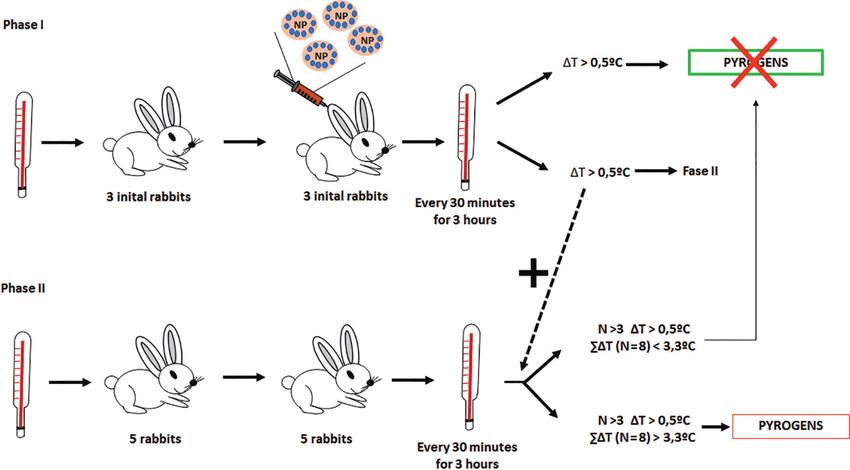 Fig 4. General scheme of the pyrogenic test. (Romero-Castillo, L., et al.; 2017)
Fig 4. General scheme of the pyrogenic test. (Romero-Castillo, L., et al.; 2017)
The Rabbit Pyrogen Test (RPT) is an in vivo methodology that is employed in the field of biomedicine for the detection of pyrogenicity. Pyrogens are subcellular entities incriminated in inducing febrile responses upon entry into the human body, common in numerous pharmaceutical preparations. For the purpose of RPT, the selected rabbit specimens should not have been part of any other experimental process for the minimal period of two weeks pre-test, ensuring their temperature range adheres to normalcy.
3. Monocyte Activation Test (MAT)
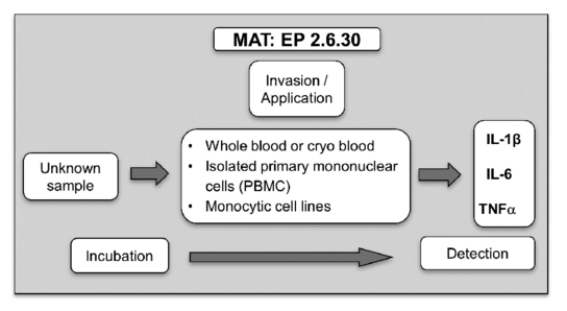 Fig 5. The biological principle of the "Monocyte Activation Test" as defined by the monograph 2.6.30 in the European Pharmacopoeia (EDQM, 2010). (Nina, H., et al.; 2013)
Fig 5. The biological principle of the "Monocyte Activation Test" as defined by the monograph 2.6.30 in the European Pharmacopoeia (EDQM, 2010). (Nina, H., et al.; 2013)
The Monocyte Activation Test (MAT) serves as a robust in vitro methodology employed to detect pyrogens and bacterial endotoxins in pharmaceutical commodities, providing an alternative to traditional rabbit pyrogen tests. A central premise of MAT hinges on the fact that human monocytes secrete a group of signaling proteins—cytokines—upon pyrogenic stimulation. Outlined next are the sequential stages involved in employing MAT for bacterial endotoxins: monocyte culture, sample preparation, sample addition, incubation, cytokine measurement, result interpretation.
Why Us?
Our Commitment and Future Goals
Creative Proteomics, a distinguished leader in the science services sector, takes immense pleasure in providing an extensive and trustworthy bacterial endotoxins testing service accommodated to the exclusive requirements and challenges of diverse sectors. We encourage inquiries and open lines of communication with our team.
References
- Yang, X., et al.; Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. Immunity. 2019.
- Galanos, C., & Freudenberg, M. A. Bacterial endotoxins: biological properties and mechanisms of action. Mediators of Inflammation. 1993, 2(7), S11–S16.
- Sandle, T. Endotoxin and pyrogen testing. Pharmaceutical Microbiology. 2016, 131–145.
- Romero-Castillo, L., et al.; Exploring the in vivo toxicity of nanoparticles. Canadian Journal of Chemistry. 2017, 95(9), 917–926.
- Nina, H., et al.; Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. Alternatives to Animal Experimentation. 2013;30(2):169-208.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. (Yang, X., et al.; 2019)
Fig 1. Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. (Yang, X., et al.; 2019) Fig 2. Risk analysis for HCP control during bioprocess development. (Galanos, C., & Freudenberg, M. A.; 1993)
Fig 2. Risk analysis for HCP control during bioprocess development. (Galanos, C., & Freudenberg, M. A.; 1993) Fig 3. LAL-clotting cascade. (Sandle, T.; 2016)
Fig 3. LAL-clotting cascade. (Sandle, T.; 2016) Fig 4. General scheme of the pyrogenic test. (Romero-Castillo, L., et al.; 2017)
Fig 4. General scheme of the pyrogenic test. (Romero-Castillo, L., et al.; 2017) Fig 5. The biological principle of the "Monocyte Activation Test" as defined by the monograph 2.6.30 in the European Pharmacopoeia (EDQM, 2010). (Nina, H., et al.; 2013)
Fig 5. The biological principle of the "Monocyte Activation Test" as defined by the monograph 2.6.30 in the European Pharmacopoeia (EDQM, 2010). (Nina, H., et al.; 2013)
