Protein sulfation is a post-translational modification of reactive cysteine residues that modulates the structure and function of proteins. Creative Proteomics provides protein sulfation services to help global customers gain a deeper understanding of cellular metabolism processes and mechanisms of aging, as well as to meet the requirements for biologics modification identification in the ICH Q6B guidelines.
Overview
Protein sulfation refers to the process of adding sulfate groups (-SO4) to proteins. Sulfate groups are negatively charged moieties that can be attached to specific amino acid residues, such as tyrosine and serine, through a post-translational modification known as sulfation.
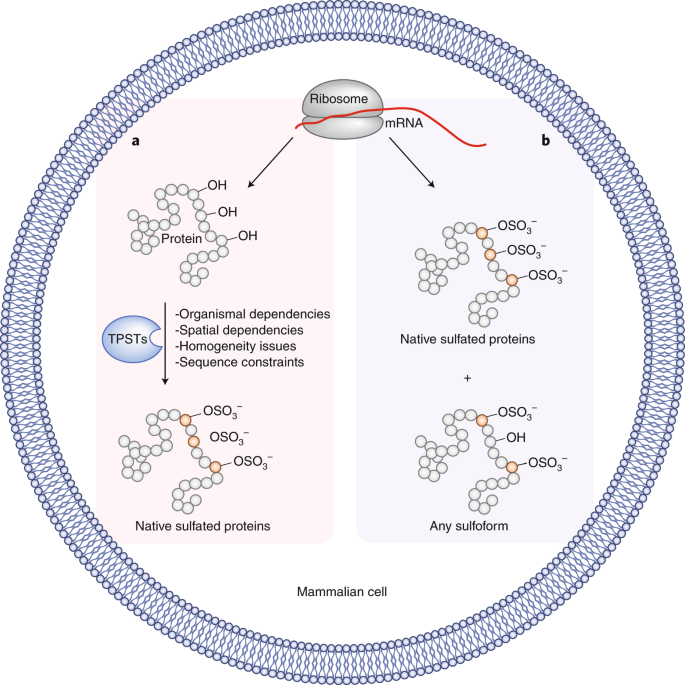 Fig 1. A new path to tyrosine sulfation. (Liu, C. C.; 2020)
Fig 1. A new path to tyrosine sulfation. (Liu, C. C.; 2020)
This modification is catalyzed by enzymes called sulfotransferases that transfer the sulfate group from a donor molecule, typically 3'-phosphoadenosine 5'-phosphosulfate (PAPS), to the target amino acid of the protein. Protein sulfation occurs in various tissues and organs and is involved in several physiological processes.
Tyrosine-sulfated Human Protein
To our knowledge, a peptide made from fibrinogen is where protein tyrosine sulfation was first noted more than 40 years ago. Tyrosine-sulfated proteins, which comprise various compounds including choriogonadotropin and heparin cofactor II, were first identified in the middle of the 1980s. A lot of these proteins with tyrosine sulfation engage in protein-protein interactions that appear to be influenced, at least in part, by the sulfate group itself. Tyrosine sulfation has been discovered as a crucial regulator of protein-protein interactions that regulate inflammatory leukocyte adhesion in recent years. Tyrosine-sulfated chemokine receptors, which were just discovered, may play an even bigger part in the inflammatory response.
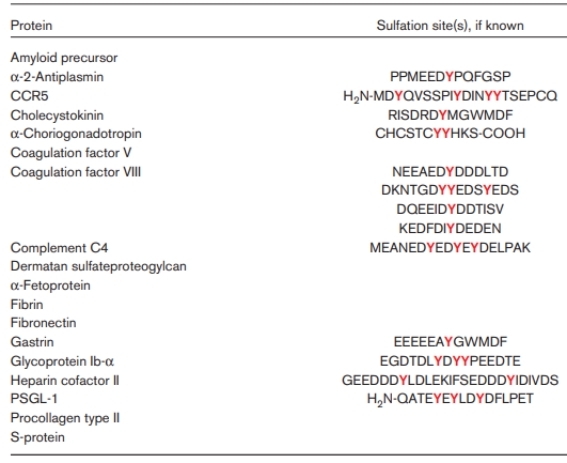 Fig 2. Human proteins known to be tyrosine sulfated. (Kehoe, J. W., & Bertozzi, C. R. 2000)
Fig 2. Human proteins known to be tyrosine sulfated. (Kehoe, J. W., & Bertozzi, C. R. 2000)
Application of Protein Sulfation
 Cell adhesion and recognition
Cell adhesion and recognition
Sulfation of certain proteins, such as selectins, integrins, and syndecans, is crucial for cell-cell and cell-matrix interactions. It enables cell adhesion, migration, and recognition processes involved in tissue development, immune response, and angiogenesis.
 Hormone function
Hormone function
Several hormones, such as thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH), undergo sulfation to enhance their activity and stability. This modification is necessary for proper hormone-receptor binding and downstream signaling.
 Proteoglycan synthesis
Proteoglycan synthesis
Sulfation is essential for the biosynthesis of proteoglycans, which are large macromolecules found in the extracellular matrix and on cell surfaces. Sulfation of glycosaminoglycans (GAGs) within proteoglycans determines their structural integrity, interaction with growth factors, and regulation of cell signaling pathways.
 Viral entry
Viral entry
Some viruses, like human immunodeficiency virus (HIV), exploit protein sulfation to facilitate their entry into target cells. Sulfated tyrosine residues on cell surface receptors, such as CD4 and CCR5, are critical for viral attachment and internalization.
 Drug development
Drug development
The understanding of protein sulfation has led to the development of drugs targeting specific sulfotransferase enzymes responsible for protein sulfation. Modulating sulfation levels can potentially influence protein function and signaling pathways, making it a target for therapeutic interventions.
Protein Sulfation Analysis
- MS is a powerful technique that can directly identify and quantify protein sulfation by analyzing the mass shifts resulting from the addition of sulfate groups.
- Immunodetection techniques, such as enzyme-linked immunosorbent assays (ELISA) or Western blotting with specific antibodies against sulfated proteins, can be used to detect protein sulfation.
- This technique involves the incorporation of radioactive sulfur isotopes into proteins during their synthesis, followed by autoradiography or scintillation counting to detect protein sulfation.
- These involve the incorporation of stable isotopes of sulfur (e.g., 34S or 18O) into proteins. After purification and digestion of the labeled proteins, mass spectrometry or other analytical methods can determine the presence and location of sulfation.
Sulfate-labeling techniques
- Various bioinformatics tools and databases have been developed to predict potential protein sulfation sites based on sequence motifs and physicochemical properties.
Our Goals?
Our clients receive cutting-edge, effective, and highly personalized protein sulfation analysis services from Creative Proteomics. We pledge to provide timely and high-quality results and goods. We are committed to supporting researchers everywhere. Feel free to get in touch with us.
References
- Liu, C. C. A new path to tyrosine sulfation. Nature Chemical Biology. 2020, 16, pages365–366
- Kehoe, J. W., & Bertozzi, C. R. Tyrosine sulfation: a modulator of extracellular protein–protein interactions. Chemistry & Biology. 2000, 7(3), R57–R61.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. A new path to tyrosine sulfation. (Liu, C. C.; 2020)
Fig 1. A new path to tyrosine sulfation. (Liu, C. C.; 2020) Fig 2. Human proteins known to be tyrosine sulfated. (Kehoe, J. W., & Bertozzi, C. R. 2000)
Fig 2. Human proteins known to be tyrosine sulfated. (Kehoe, J. W., & Bertozzi, C. R. 2000)