ICH Topic Q6B states "Other parts of this strategy include thorough product characterisation during development, upon which many of the specifications are based, adherence to Good Manufacturing Practices, a validated manufacturing process, raw materials testing, in-process testing, stability testing, etc."
Creative Proteomics offers protein Tm stability analysis services to help customers understand the structure and function of proteins and how protein stability changes with temperature. Such Tm values are commonly used to evaluate protein stability, optimize protein expression and purification processes, and perform drug screening.
Background
The thermal stability of a protein, scientifically referred to as its melting temperature (Tm), delineates the precise temperature at which a protein undergoes denaturation or unfolds. The stability of proteins is fundamentally governed by an intricate interplay of factors which include but are not limited to, hydrogen bonding, hydrophobic interactions, and electrostatic interactions. It's noteworthy that a surge in temperature can disrupt these crucial interactions. Generally speaking, a protein with a superior Tm value bespeaks enhanced stability, rendering it better equipped to endure rigorous conditions, such as elevated temperatures or extreme pH environments.
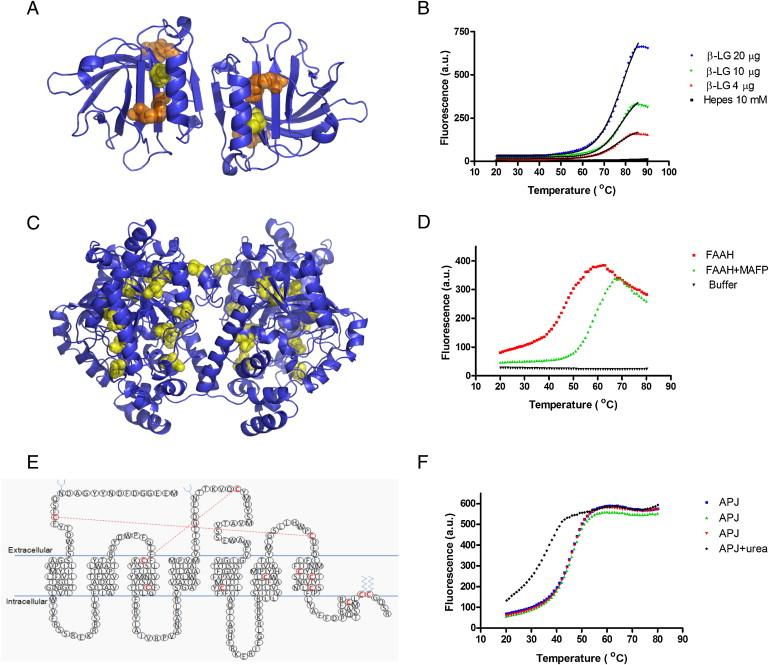 Fig 1. Thermal Stability Profiles of β-LG, FAAH, and APJ Receptor (Alexandrov, A. I., et al.; 2008)
Fig 1. Thermal Stability Profiles of β-LG, FAAH, and APJ Receptor (Alexandrov, A. I., et al.; 2008)
Why do Thermal (Tm) Stability Analysis?
 Predicting protein stability
Predicting protein stability
The implementation of Tm analysis facilitates the foresight into the stability of a protein within diverse environments. Proteins presenting higher Tm values exhibit enhanced stability levels, tolerating increases in temperature without undergoing denaturation.
 Understanding protein folding
Understanding protein folding
The unfolding process of proteins, paired with the stability of the original structure, is deciphered through Tm analysis. This provides invaluable insights into this structurally complex process.
 Characterizing protein-ligand interactions
Characterizing protein-ligand interactions
The impact of ligand binding on protein stability can be scrutinized through thermal stability analysis charts. These studies reveal modifications in Tm values pre and post ligand-binding activity, offering details concerning the binding affinity, and the sturdiness of the protein-ligand structure.
 Protein engineering and optimization
Protein engineering and optimization
The evaluation of a protein's thermal stability can reveal regions or specific residues that are the sources of destabilization. Such knowledge propels strategies in protein engineering aimed at bolstering stability, optimizing expression, augmenting functional activity, or extending shelf-life.
Our Service
1. Differential Scanning Calorimetry (DSC)

DSC denotes a technique utilized in examining changes in the heat capacity of a protein sample as a function of temperature increment. The produced curve, or thermogram, captures transitions marking the denaturation process of the protein, perceived at the melting temperature (Tm).
2. Fluorescence Spectroscopy
Fluorescence spectroscopy serves as a monitor for alterations within the protein structure and stability, using either the inherent fluorescence of proteins or fluorescent dyes. A classic application case would be the usage of SYPRO Orange, binding to hydrophobic areas exposed throughout the unfolding process of proteins, which consequently amplifies fluorescence intensity. The Tm can be computed based on evaluating the midpoint of the resulting unfolding curve.
3. Circular Dichroism (CD) Spectroscopy
CD spectroscopy conceptually relies on examining the disparity in absorbance between left- and right-circular polarized light by chiral compounds. Steps towards denaturation in secondary protein structure are detectable by shifts in CD spectra, therefore, the Tm can be deduced from studying variations within protein structures subject to the rising temperature.
4. Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectroscopy, pertinently, gauges protein sample absorption rates in conjunction with infrared light. Providing insights into changes in the secondary structure, as well as hydrogen bonding patterns within protein samples. Similarly, the Tm can be determined by probing modifications of the protein structure in response to enhanced temperature.
5. Thermal Shift Assay
Thermal shift assay encompasses a methodological approach that observes the shift in protein fluorescence or absorbance as a fluorescent or ultraviolet-absorbing dye binds to the protein. The dye's emission or absorption properties are subject to adjustments due to transformations within the protein structure. As such, the Tm may be deduced by quantifying the midpoint of the unfolding curve.
Our Advantages
- Expert Proficiency: Our firm boasts an ensemble of seasoned biologists with substantive knowledge and experience in protein stability analysis methodologies.
- Cutting-Edge Analytical Techniques: We utilize avant-garde techniques in discerning protein thermal stability; these include differential scanning calorimetry (DSC), fluorescence spectroscopy, and circular dichroism spectroscopy.
- Personalized Methodologies: We offer custom-made solutions designed to suit the unique characteristics of the protein under scrutiny. This may involve modifying the conditions of the experiment, developing bespoke protocols, or engaging complementary methods.
- High-Throughput Facilities: Our capability to analyze myriads of protein samples in tandem makes us a favored choice, especially in situations that require protein screening or pharmaceutical research endeavors.
- Comprehensive Data Analysis and Interpretation: Our firm offers extensive data analysis, interpretation, and a detailed report to aid clients in decoding the ramifications of the thermal stability findings.
- Confidentiality and Rigorous Quality Control: We hold client confidentiality in high regard and maintain strict adherence to rigorous quality control standards throughout the course of the analysis process.
Service Workflow
Want to Learn More?
As a well-established entity in the field of proteomics, Creative Proteomics specializes in providing in-depth protein thermal stability analysis services of exceptional quality that facilitate research and development across numerous disciplines. Our primary objective is to support our clientele in gaining insightful understanding of protein thermal stability, its optimization and in leveraging this knowledge towards the invention of novel pharmaceutical applications and industrial uses. We welcome interaction and inquiries from all interested parties regarding our services.
Reference
- Alexandrov, A. I., et al.; Microscale Fluorescent Thermal Stability Assay for Membrane Proteins. Structure. 2008, 16(3), 351–359.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Thermal Stability Profiles of β-LG, FAAH, and APJ Receptor (Alexandrov, A. I., et al.; 2008)
Fig 1. Thermal Stability Profiles of β-LG, FAAH, and APJ Receptor (Alexandrov, A. I., et al.; 2008)





