Creative Proteomics stands as a pre-eminent entity offering an array of scientific research services with a focus on protein biosafety analysis, a specialized field in biology. These investigative analyses are conducted within the framework of the ICH Q6B guidelines, ensuring adherence to stringent quality and safety standards.
Overview of Protein Biosafety
Biosafety in protein handling constitutes an array of precautionary routines and normative instructions put in place whilst interacting with proteins that hold potential biological risks. It circumscribes strategic practices to avert exposure to potentially detrimental proteins, efficacious administration and regulation of biohazards, proficient utilization and manipulation of proteins, and the education of scholars or professionals about safety protocols. The prime objective resides in the safeguarding of individuals, the larger society, and the environment from possible detrimental impacts. These safety measures assume significant importance in sectors including but not limited to healthcare, pharmaceuticals, food production, agriculture, and scientific inquiry.
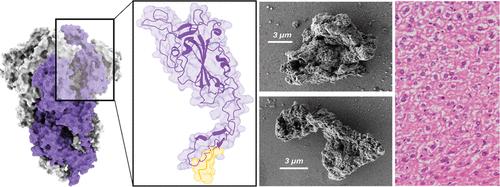 Fig 1. Probing the Biosafety of Implantable Artificial Secretory Granules for the Sustained Release of Bioactive Proteins. (Álamo, Patricia, et al. 2023)
Fig 1. Probing the Biosafety of Implantable Artificial Secretory Granules for the Sustained Release of Bioactive Proteins. (Álamo, Patricia, et al. 2023)
Why do Protein Biosafety Analysis?
Analysis of protein biosafety is paramount in evaluating potential hazards that may arise from protein utilization, specifically in the realm of therapeutics and diagnostics. There are several key reasons why protein biosafety analysis is conducted:
 The initial rationale behind conducting a risk assessment of protein is the ability of this analysis to recognize potential dangers associated with protein use. Recognizing these risks takes precedence over other considerations as it is critical to ensuring patient safety and preserving a benign environment.
The initial rationale behind conducting a risk assessment of protein is the ability of this analysis to recognize potential dangers associated with protein use. Recognizing these risks takes precedence over other considerations as it is critical to ensuring patient safety and preserving a benign environment.
 Additionally, the regulatory authorities necessitate a thorough safety evaluation of proteins prior to their approval for use in humans or animals. The role of protein biosafety analysis in this context is to ensure adherence to stipulated regulatory guidelines and ease the process of obtaining permissions necessary for the commercialization of protein-oriented products.
Additionally, the regulatory authorities necessitate a thorough safety evaluation of proteins prior to their approval for use in humans or animals. The role of protein biosafety analysis in this context is to ensure adherence to stipulated regulatory guidelines and ease the process of obtaining permissions necessary for the commercialization of protein-oriented products.
 Furthermore, monitoring protein consistency and quality is another dominant function of protein biosafety analysis. It is crucial in identifying and eradicating contaminants, impurities, or other factors that may compromise the efficacy or safety of the product.
Furthermore, monitoring protein consistency and quality is another dominant function of protein biosafety analysis. It is crucial in identifying and eradicating contaminants, impurities, or other factors that may compromise the efficacy or safety of the product.
Our Service
Creative Proteomics, a premier analytical service provider, is adept at rendering comprehensive bacterial endotoxins testing service, proficiently catering to the demands of pharmaceutical, biotechnological and, medical device industries. Creative Proteomics is resolutely committed to proffering superior testing services, whether for the detection and quantification of endotoxins or evaluating product purity and safety.
Creative Proteomics provides an exceptionally proficient bioburden testing service, meticulously crafted to quantify the entire microbial population present within any given sample. The primary objective here is to guarantee the safety, cleanliness, and freedom from excessive bacteria, yeast, mold, and other microbial infections of the products.
Creative Proteomics is a broad-based company focused on life sciences and pharmaceuticals that offers highly comprehensive sterility testing services. Ensuring sterility and absence of microbial contamination in biological materials that includes cell banks, raw materials, and final products, is a critically imperative task their service handles.
Creative Proteomics is a firm renowned for its specialization in proteomics services. One particularly interesting service we offer is dedicated to the testing of abnormal toxicity. This essential service holds invaluable significance in guaranteeing the safety and effectiveness of a wide range of products, focusing predominantly on those found within the medical and pharmaceutical sectors.
Creative Proteomics, an internationally acclaimed entity noted for its superior proteomics operations, provides a wide-ranging mycoplasma testing service. As mycoplasma contamination bears the potential to adversely influence the integrity of cell lines and resulting research findings, this service fulfills a crucial role in safeguarding the quality assurance of scientific exploration and production processes through early detection of any contamination.
Creative Proteomics is a globally recognized company specializing in the provision of advanced scientific services, including the critical service of subvisible particles analysis. This service is key for several sectors that deal with nanoparticle analysis, including biopharmaceuticals, cosmetics, and nanotechnology.
Creative Proteomics is a renowned service provider that specializes in the extensive field of proteomics and genomics, and it offers a unique service known as visible particles analysis service. This includes, but is not limited to, the pharmaceutical industry, where visible particle analysis is essential for quality control and safety purposes to ensure there are no unwanted particles present in the drugs produced.
Sample Requirement
| Sample Type |
Protein |
Cell |
Animal tissue |
Plant Tissue |
Blood (EDTA added) |
Serum |
Urine |
Microbes |
| Quantity |
100 ug |
2X107 cells |
1g |
200 mg |
1mL |
0.2-0.5 mL |
2 mL |
Dry weighed: 200 mg |
Service Process

Want to Learn More?
As a reputable entity in the realm of proteomics, Creative Proteomics provides a specialized service called protein biosafety analysis. This service is uniquely designed for researchers and pharmaceutical enterprises involved in protein-centric studies and who are keen to understand potential risks associated with the use of specific proteins. We are accessible for further discussions or clarifications related to our services.
Reference
- Álamo, Patricia, et al. Probing the Biosafety of Implantable Artificial Secretory Granules for the Sustained Release of Bioactive Proteins. ACS Applied Materials & Interfaces. 2023.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Probing the Biosafety of Implantable Artificial Secretory Granules for the Sustained Release of Bioactive Proteins. (Álamo, Patricia, et al. 2023)
Fig 1. Probing the Biosafety of Implantable Artificial Secretory Granules for the Sustained Release of Bioactive Proteins. (Álamo, Patricia, et al. 2023)
