Creative Proteomics is a renowned and leading company that specializes in providing exceptional protein physicochemical property determination services. By availing our services, clients can gain valuable insights into the physicochemical attributes of proteins, which can aid in various scientific fields such as drug discovery, personalized medicine, and biomarker identification.
What is Protein Physicochemical Property?
ICH Topic Q6B states "A physicochemical characterisation program will generally include a determination of the composition, physical properties, and primary structure of the desired product. In some cases, information regarding higher-order structure of the desired product (the fidelity of which is generally inferred by its biological activity) may be obtained by appropriate physicochemical methodologies."
The "physicochemical properties" of proteins are their physical and chemical characteristics that affect their structure, stability, and function and are extremely important in determining how they behave in biological systems. Studying the structure, function, and behavior of proteins in various biological processes requires an understanding of their physical and chemical properties. It also aids in the modification or optimization of proteins for specific applications in protein engineering and drug design.
Some common protein physicochemical properties include:

Protein Physicochemical Property Mehodology
1. Isoelectric focusing (IEF)
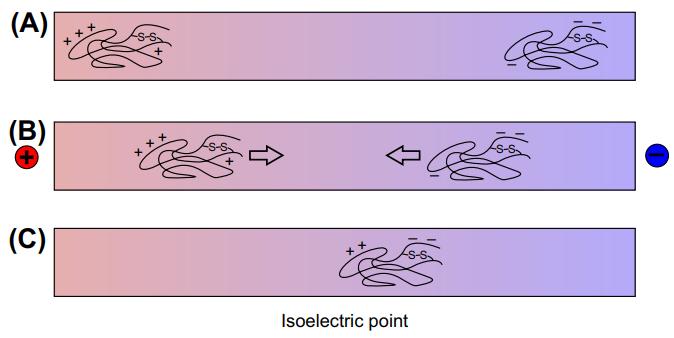 Fig 1. Isoelectric focusing. (Drabik, A., et al.; 2016)
Fig 1. Isoelectric focusing. (Drabik, A., et al.; 2016)
Isoelectric focusing (IEF) is a widely used method to determine the isoelectric point (pI) of a protein. The pI is the pH at which a protein has no net charge and when placed in an electric field, it remains stationary. To detect the pI by IEF, a protein sample is loaded onto a pH gradient gel or strip. An electric current is then applied to the gel, causing the proteins to migrate towards their respective pI values. The proteins will stop migrating when they reach regions of the gel with a pH equal to their pI.
2. Thioflavin T (ThT) fluorescence assay
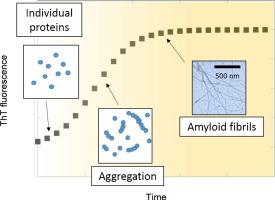 Fig 2. Agitation of amyloid proteins to speed aggregation measured by ThT fluorescence. (Batzli, K. M., & Love, B. J.; 2015)
Fig 2. Agitation of amyloid proteins to speed aggregation measured by ThT fluorescence. (Batzli, K. M., & Love, B. J.; 2015)
Thioflavin T (ThT) fluorescence assay is commonly used to study protein aggregation, particularly the formation of amyloid fibrils. ThT is a small molecule and belongs to a class of compounds termed thioflavins. It exhibits enhanced fluorescence when bound to aggregated protein structures, specifically amyloid fibrils. ThT fluorescence assay takes advantage of the unique binding properties of ThT to these amyloid structures. ThT binds to the β-sheet rich conformation of amyloid fibrils, resulting in a red-shift in its emission spectrum and an increase in fluorescence intensity. This assay is highly sensitive and specific to amyloid fibril formation due to the unique binding of ThT to these structures.
3. Differential scanning calorimetry
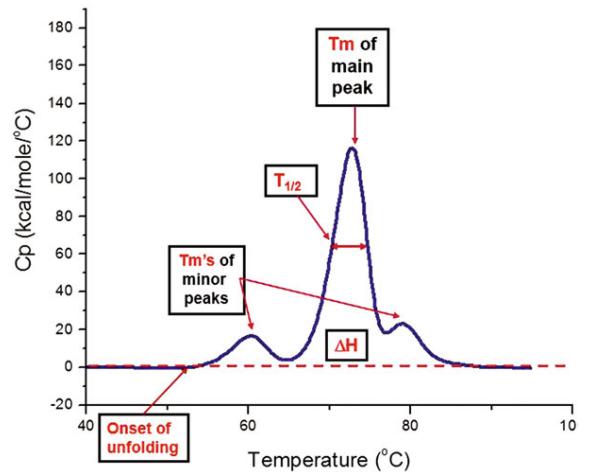 Fig 3. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Bowers, K., & Markova, N.; 2019)
Fig 3. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Bowers, K., & Markova, N.; 2019)
The principle of thermal stability emphasizes the ability of a material to continue its structural integrity under elevated temperatures, resisting decomposition or degradation. A key analytical technique employed to ascertain this characteristic among a range of other thermal properties is Differential Scanning Calorimetry (DSC). DSC has emerged as a vital instrument in examining the heat flow interactions with a material while it experiences an alteration in the phase, a chemical reaction or any such thermal event. Events may encompass processes such as melting, crystallization, vitrification and decomposition. The assessment of this heat flow data furnishes vital information about a material's thermal stability, hence underlining the utility of DSC in the understanding of material composition and reactions.
4. Bicinchoninic acid (BCA) assay
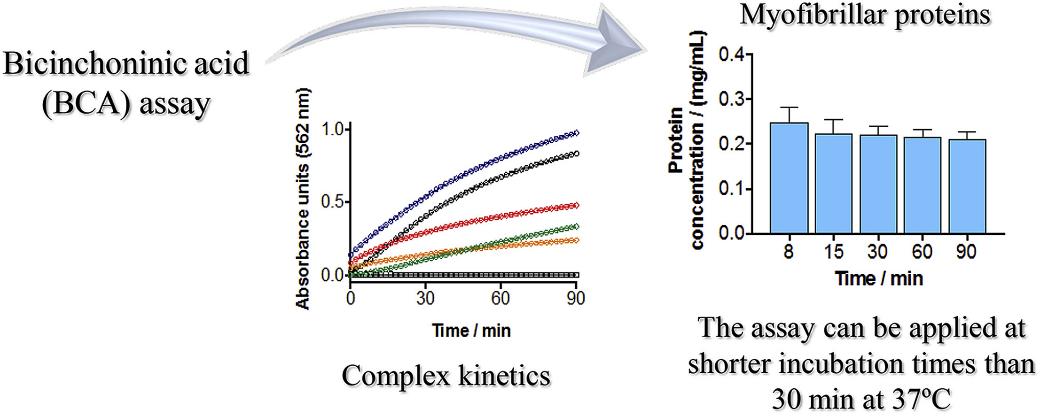 Fig 4. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Cortés-Ríos, J., et al.; 2020)
Fig 4. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Cortés-Ríos, J., et al.; 2020)
As an authority in a biology-related field, I can affirm that the bicinchoninic acid (BCA) assay is a widely utilized method for quantifying protein levels. This technique capitalizes on the proteins' inherent ability to reduce Cu2+ ions to Cu1+. The Cu1+ ions subsequently react with bicinchoninic acid, resulting in the formation of a purple-colored complex. The direct proportionality of this complex to the protein concentration within the test sample allows for quantification through spectrophotometry. Predominantly in biochemical, medicinal, and pharmaceutical research contexts, the BCA assay is employed extensively to approximate protein concentrations within a variety of sample types. Its effectiveness and simple application make it particularly beneficial in high-throughput analysis scenarios.
What can We Provide for Protein Physicochemical Property?
Creative Proteomics offers comprehensive protein isoelectric point determination services. Our competence can handle any sample type, including complex biological matrices. Depending on the needs of the customer, Creative Proteomics can provide both qualitative and quantitative analysis of protein isoelectric points. Our company also offers bespoke services, allowing clients to tailor the study to their own needs.
Creative Proteomics provides a range of services for protein charge variant analysis, and offers advanced technologies and techniques to identify and characterize various charge variants present in proteins. Creative Proteomics employs state-of-the-art equipment and employs highly experienced scientists to ensure accurate and reliable analysis of protein charge variants. We also provide customized solutions to meet the specific needs and requirements of their clients in terms of charge variant analysis of proteins.
At Creative Proteomics, we provide comprehensive services for determining protein extinction coefficients employing a diverse set of robust techniques. These methodologies include UV-Vis Spectroscopy, quantitative amino acid composition analysis, and peptide mapping. Through UV-Vis spectroscopy, we quantify the absorption of light of various wavelengths by a protein solution. Additionally, we conduct an extensive amino acid composition analysis. Here, we determine the relative concentrations of the constituent amino acids within a specific protein. Lastly, through peptide map analysis, we are able to identify and quantify the individual amino acids that make up a protein. As a specialist in the field, I can confirm these techniques are integral in accurately assessing the absorption properties of proteins, a fundamental aspect in various biological and biochemical research.
At Creative Proteomics, we specialize in the elucidation and characterization of protein aggregates via a suite of advanced analytical techniques encompassing gel electrophoresis, size exclusion chromatography, and dynamic light scattering. These techniques allow us to accurately determine the size, elemental composition, and stability characteristics of the aggregates in question. Alongside this, we offer a cutting-edge propensity analysis service designed to forecast protein aggregation behaviors. In particular, we boast extensive experience in conducting aggregation studies for a diverse range of protein therapeutic candidates. This aids in assessing the implications of various formulation and production processes on protein aggregation. To ensure ultimate customer satisfaction and optimization of results, we can customize our services according to the discrete requirements of our clients.
Creative Proteomics leads the field in targeted protein degradation analysis, offering in-depth evaluation of target protein break down, along with identification of their resulting byproducts. Furthermore, we encompass global protein degradation assessments, which involve the identification of degradation byproducts and the understanding of their respective degradation pathways. Our services are conducted using state-of-the-art methodologies, leveraging advanced technologies such as mass spectrometry, proteomics, and bioinformatics. In our commitment to excellence, we provide clients with a comprehensive exploration and detailed reports on the outcomes of our protein degradation analysis.
Creative Proteomics' services include various approaches to study the thermal stability of proteins, such as thermal shift assays, differential scanning fluorimetry (DSF), and temperature ramping assays. Our experienced team utilizes advanced techniques and equipment to evaluate protein stability under various conditions, including buffer optimization, thermal denaturation, and ligand binding. Through these services, Creative Proteomics aims to help researchers understand the behavior and stability of proteins, which is critical for various applications in drug discovery and protein engineering.
Creative Proteomics provides a comprehensive range of services for protein quantitation, catering to the diverse needs of researchers. Our protein quantification assays can detect different ranges of proteins, including ultraviolet absorbance at 280 nm (Range: 20–3000 μg), ultraviolet absorbance at 205 nm (Range: 1–100 μg), lowry (Alkaline Copper Reduction Assays) (Range: 5–100 μg), bicinchoninic Acid (BCA) (Range: 0.2–50 μg), amine Derivatization (Range: 0.05–25 μg) and detergent-Based Fluorescent Detection (Range: 0.02–2 μg). Creative Proteomics ensures accuracy, sensitivity, and repeatability through these advanced quantification techniques.
Sample Requirement
| Sample Type |
Protein |
Cell |
Animal tissue |
Plant Tissue |
Blood (EDTA added) |
Serum |
Urine |
Microbes |
| Quantity |
100 ug |
2X107 cells |
1g |
200 mg |
1mL |
0.2-0.5 mL |
2 mL |
Dry weighed: 200 mg |
Service Workflow
References
- Drabik, A., et al.; Gel Electrophoresis. Proteomic Profiling and Analytical Chemistry. 2016, 115–143.
- Batzli, K. M., & Love, B. J. Agitation of amyloid proteins to speed aggregation measured by ThT fluorescence: A call for standardization. Materials Science and Engineering: C. 2015, 48, 359–364.
- Bowers, K., & Markova, N. Value of DSC in Characterization and Optimization of Protein Stability. Microcalorimetry of Biological Molecules. 2019, 33–44.
- Cortés-Ríos, J., et al.; Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Analytical Biochemistry. 2020, 113904.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.



 Fig 1. Isoelectric focusing. (Drabik, A., et al.; 2016)
Fig 1. Isoelectric focusing. (Drabik, A., et al.; 2016) Fig 2. Agitation of amyloid proteins to speed aggregation measured by ThT fluorescence. (Batzli, K. M., & Love, B. J.; 2015)
Fig 2. Agitation of amyloid proteins to speed aggregation measured by ThT fluorescence. (Batzli, K. M., & Love, B. J.; 2015) Fig 3. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Bowers, K., & Markova, N.; 2019)
Fig 3. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Bowers, K., & Markova, N.; 2019) Fig 4. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Cortés-Ríos, J., et al.; 2020)
Fig 4. Buffer-subtracted and baseline-corrected DSC thermogram of a mAb protein with key stability metrics highlighted. (Cortés-Ríos, J., et al.; 2020)
