To meet the requirements of the ICH Q6B guidelines, Creative Proteomics offers a free sulfhydryl analysis service to assist researchers in determining proteins that have undergone structural changes, as well as exposure to oxidation during storage, handling or use.
What is Free Sulfhydryl?
The free sulfhydryl group, commonly abbreviated as -SH or thiol group, is a functional group made composed of an atom of sulfur linked to an atom of hydrogen. Because it does not take part in disulfide bonding (S-S) or any other covalent bonds, it is referred to as being "free". The free sulfhydryl group is often quite reactive and can take part in a wide range of chemical processes, including bond formation, oxidation, and reduction. Free sulfhydryl groups are essential for enzyme activity, protein structure and function, and redox signaling in a biological setting.
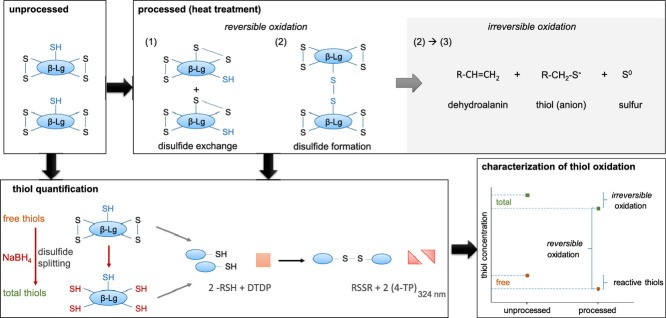 Fig 1. RP-HPLC method for simultaneous quantification of free and total thiol groups. (Kurz, F., Hengst, C., & Kulozik, U. 2020)
Fig 1. RP-HPLC method for simultaneous quantification of free and total thiol groups. (Kurz, F., Hengst, C., & Kulozik, U. 2020)
The production of disulfide bonds, which are extremely stable covalent connections that improve the three-dimensional structural stability and function of proteins, is the most significant role that sulfhydryl groups play in biology. If the presence of free sulfhydryl groups is detected during protein isolation, it indicates that the protein may have undergone partial reduction because sulfhydryl groups usually form disulfide bonds with other chemicals and are anchored in the protein structure, and free sulfhydryl groups indicate that these disulfide bonds have been broken. This could mean that the protein has been oxidised or otherwise affected, resulting in changes to its structure.
Characterizing Free Sulfhydryl Analysis
Ellman's Assay
This is a commonly used method for the quantification of free sulfhydryl groups. The method involves the reaction of sulfhydryl groups with a specific reagent called 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) or Ellman's reagent. The reaction produces a yellow colored product that can be measured spectroscopically. The absorbance is directly proportional to the concentration of free sulfhydryl groups.
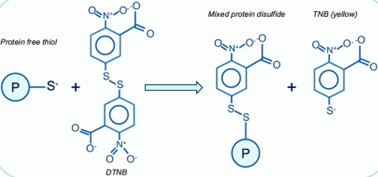 Fig 2. Detection of the reduced (free) thiol content. (Rudyk, O., & Eaton, P. 2014)
Fig 2. Detection of the reduced (free) thiol content. (Rudyk, O., & Eaton, P. 2014)
Thiol-Disulfide Exchange
This method involves the disruption of disulfide bonds and subsequent quantification of the released sulfhydryl groups. This can be achieved by using reducing agents like dithiothreitol (DTT) or tris(2-carboxyethyl)phosphine (TCEP). The concentration of free sulfhydryl groups can then be determined using the Ellman's assay or other methods.
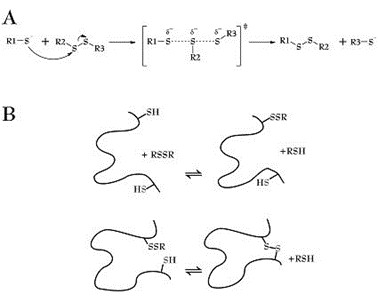 Fig 3. Thiol-disulfide exchange mechanism. (Ágoston, V., et al.; 2005)
Fig 3. Thiol-disulfide exchange mechanism. (Ágoston, V., et al.; 2005)
Fluorescent Labeling
Various fluorescent probes are available that can selectively label sulfhydryl groups. Once labeled, the fluorescence intensity can be measured using spectroscopic techniques to quantify the sulfhydryl groups. Examples of such probes include maleimide-based dyes like 5-iodoacetamidofluorescein (IAF) or 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABD-F).
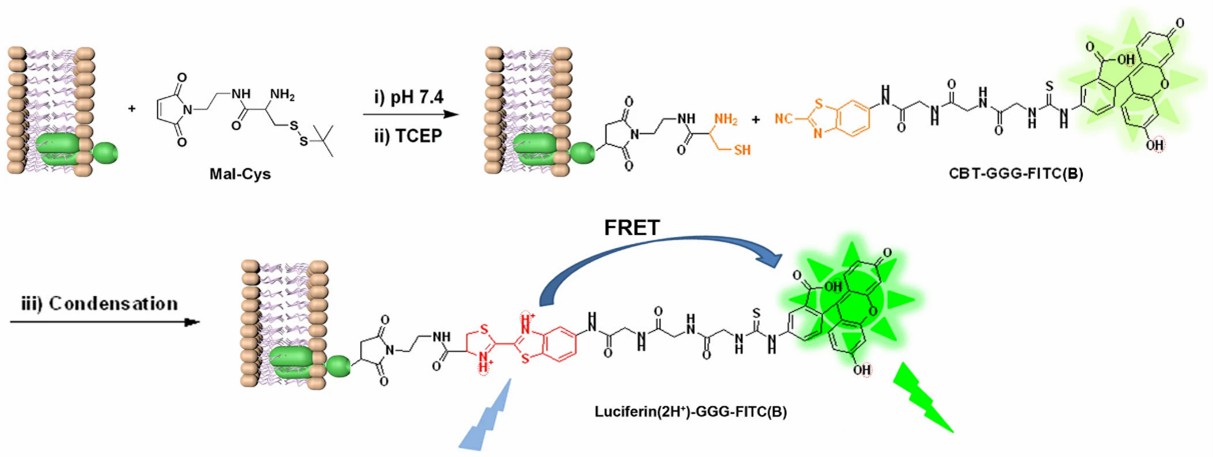 Fig 4. Labeling Thiols on Proteins, Living Cells and Tissues with Enhanced Emission Induced by FRET. (Yuan, Y., et al.; 2013)
Fig 4. Labeling Thiols on Proteins, Living Cells and Tissues with Enhanced Emission Induced by FRET. (Yuan, Y., et al.; 2013)
Mass Spectrometry
Mass spectrometry can also be employed for the analysis of disulfide bonds. Techniques like tandem mass spectrometry (MS/MS) or top-down proteomics can be useful for characterizing and identifying disulfide bonds in proteins.
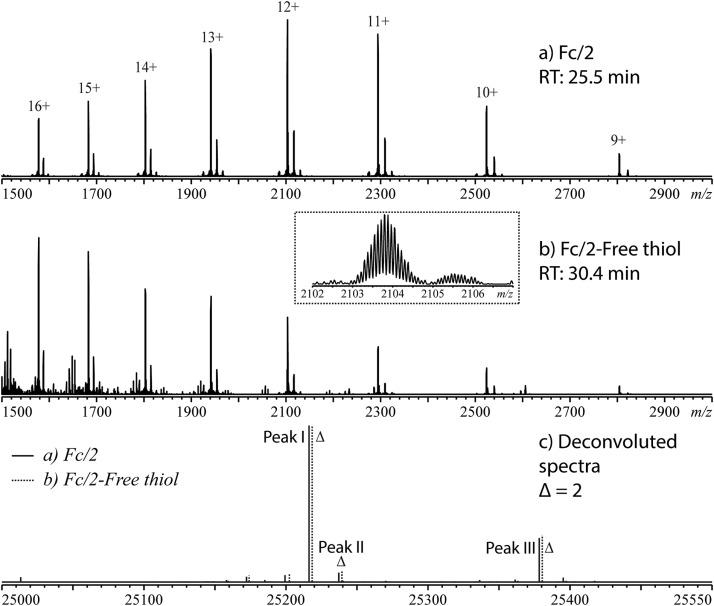 Fig 5. Mass spectra of (a) Fc/2 main peak and (b) one of the Fc/2 free thiol peaks of mAb-A, as well as (c) the deconvoluted mass spectra overlaid. (Pu, Y., et al.; 2019)
Fig 5. Mass spectra of (a) Fc/2 main peak and (b) one of the Fc/2 free thiol peaks of mAb-A, as well as (c) the deconvoluted mass spectra overlaid. (Pu, Y., et al.; 2019)
What We Provide?
- Quantify the amount of free sulfhydryl
- Study the kinetics of oxidation of free sulfhydryl
- Evaluate the efficiency in protecting free sulfhydryl
- Investigate the reactivity and exchange dynamics of free sulfhydryl
- Determine the redox potential of free sulfhydryl
- Analyze the modification of free sulfhydryl
- Develop and perform biochemical assays to investigate the functional significance of free sulfhydryl
Why Choose Creative Proteomics's Free Sulfhydryl Analysis Service?

State-of-the-art Technology

Expert Team

Data Confidentiality

Customized Solutions
Our Goals
Free sulfhydryl analysis plays a crucial role in understanding the functional properties and pharmaceutical potential of protein-based drugs. Creative Proteomics offers a range of services, including free sulfhydryl analysis service, to accurately determine protein structure and aid in drug development. With our state-of-the-art technology, expert team, and commitment to confidentiality, we are dedicated to providing customized solutions and driving advancements in the field. Join us in unlocking the mysteries of protein structure to pave the way for novel therapeutics and improved drug design.
Please feel free to contact us and we look forward to working with you on attractive projects.
References
- Kurz, F., Hengst, C., & Kulozik, U. RP-HPLC method for simultaneous quantification of free and total thiol groups in native and heat aggregated whey proteins. MethodsX. 2020, 7, 101112.
- Rudyk, O., & Eaton, P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biology. 2014, 2, 803–813.
- Ágoston, V., et al.; Graph-representation of oxidative folding pathways. BMC Bioinformatics. 2005, 6(1), 19.
- Yuan, Y., et al.; Labeling Thiols on Proteins, Living Cells and Tissues with Enhanced Emission Induced by FRET. Scientific Reports. 2013, 3(1).
- Pu, Y., et al.; Application of a label-free and domain-specific free thiol method in monoclonal antibody characterization. Journal of Chromatography B. 2019, 1114-1115, 93–99.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. RP-HPLC method for simultaneous quantification of free and total thiol groups. (Kurz, F., Hengst, C., & Kulozik, U. 2020)
Fig 1. RP-HPLC method for simultaneous quantification of free and total thiol groups. (Kurz, F., Hengst, C., & Kulozik, U. 2020) Fig 2. Detection of the reduced (free) thiol content. (Rudyk, O., & Eaton, P. 2014)
Fig 2. Detection of the reduced (free) thiol content. (Rudyk, O., & Eaton, P. 2014) Fig 3. Thiol-disulfide exchange mechanism. (Ágoston, V., et al.; 2005)
Fig 3. Thiol-disulfide exchange mechanism. (Ágoston, V., et al.; 2005) Fig 4. Labeling Thiols on Proteins, Living Cells and Tissues with Enhanced Emission Induced by FRET. (Yuan, Y., et al.; 2013)
Fig 4. Labeling Thiols on Proteins, Living Cells and Tissues with Enhanced Emission Induced by FRET. (Yuan, Y., et al.; 2013) Fig 5. Mass spectra of (a) Fc/2 main peak and (b) one of the Fc/2 free thiol peaks of mAb-A, as well as (c) the deconvoluted mass spectra overlaid. (Pu, Y., et al.; 2019)
Fig 5. Mass spectra of (a) Fc/2 main peak and (b) one of the Fc/2 free thiol peaks of mAb-A, as well as (c) the deconvoluted mass spectra overlaid. (Pu, Y., et al.; 2019)



