Comprehensive glycosylation characterisation of glycoproteins is required by the ICH Topic Q6B guidelines. Understanding glycosylation site occupancy is crucial for comprehending protein function and regulatory mechanisms since glycosylation can impact the stability, activity, interactions, and other properties of proteins.The percentage of glycosylation sites utilized in a glycosylation reaction can be ascertained using Creative Proteomics' protein glycosylation site occupancy analysis service.
Glycosylation Site Occupancy
Glycosylation site occupancy refers to the percentage of protein molecules in a sample that are glycosylated at a specific site or sites. Glycosylation is the enzymatic process by which sugar molecules are attached to proteins, forming glycoproteins. The occupancy level indicates how many of the potential glycosylation sites on a protein are actually occupied by sugar molecules. It is important to determine glycosylation site occupancy as it affects the structure, stability, function, and immunogenicity of glycoproteins.
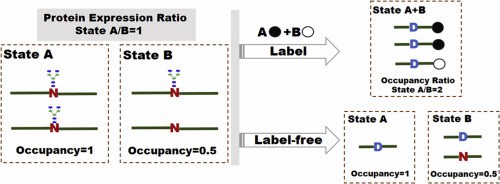 Fig 1. MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang, S., et al.;)
Fig 1. MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang, S., et al.;)
Glycosylation Site Occupancy Analysis Methodology
Liquid Chromatography-Mass Spectrometry (LC-MS)
This is the most widely used method for determining glycosylation site occupancy. It involves the separation of peptides via liquid chromatography and the analysis of their mass-to-charge ratio via mass spectrometry.
Tandem Mass Spectrometry (MS/MS)
In this method, peptides are first fragmented into smaller pieces in the mass spectrometer. The fragments are then analyzed to obtain information about the sequence and modifications of the peptide.
Nuclear magnetic resonance (NMR)
This technique relies on the magnetic properties of certain atomic nuclei and can provide detailed information about the structure and dynamics of proteins.
Enzymatic Digestion followed by Mass Spectrometry
In this method, proteins are digested into peptides using specific enzymes. The peptides are then analyzed by mass spectrometry to identify and quantify the glycosylation sites.
Matrix-Assisted Laser Desorption/Ionization (MALDI)
The samples are mixed with appropriate matrix materials and irradiated with a laser to produce ions, which are then separated and analyzed to determine the occupancy of glycosylation sites.
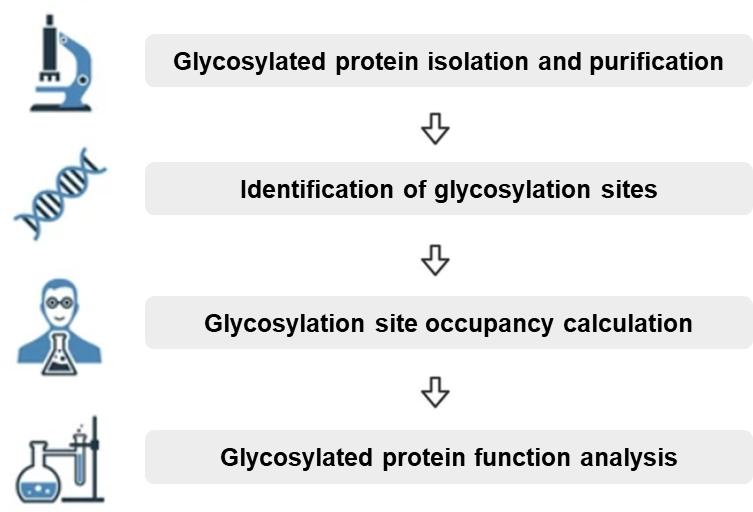
Workflow for Quantitative Glycosylation Site Occupancy Analysis
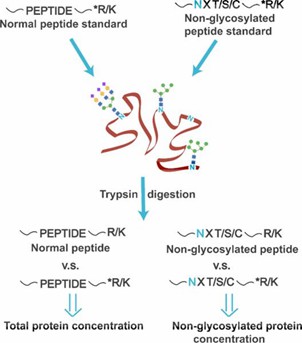 Fig 2. Peptide standards with heavy isotope-labeled tryptic termini (denoted as *R/K) are spiked to quantify the total protein concentration and the non-glycosylated protein concentration (Zhu, Z., et al.; 2014)
Fig 2. Peptide standards with heavy isotope-labeled tryptic termini (denoted as *R/K) are spiked to quantify the total protein concentration and the non-glycosylated protein concentration (Zhu, Z., et al.; 2014)
Prior to trypsin digestion, isotopically labeled internal standards are introduced into the glycoprotein sample, and the digested mixture is examined by LC-MS. Equation 1 yields the site occupancy for a particular N-glycosylation site:
Site Occupancy% = (Site-Occupied Protein Concentration) / (Total Protein Concentration×100).…...…………………………………..(1)
Additionally, the complete protein population could be split into two groups: those with occupied glycosylation sites and those without any such sites:
Total Protein Concentration − Site-Occupied Protein Concentration + Site-Unoccupied Protein Concentration……………………………(2)
Equation 3 is used to calculate the site occupancy by combining equations 1 and 2:
Site Occupancy% = [1−(Site-Unoccupied Protein Concentration) /Total Protein Concentration] ×100……………………………………….(3)
What can we offer?
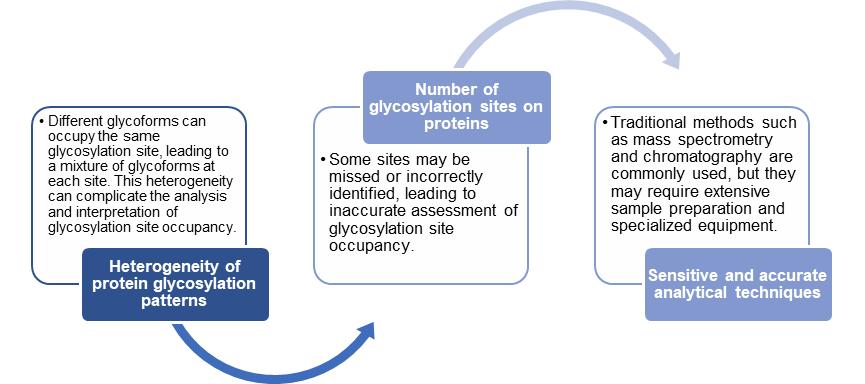
Challenges in Glycosylation Site Occupancy Analysis
The challenges in glycosylation site occupancy analysis lie in the complexity and heterogeneity of glycosylation patterns, the technical limitations of analytical techniques, and the statistical analysis of comparative data.
Our Commitment and Future Goals
When characterizing protein drugs, glycosylation site occupancy analysis is crucial because it offers important details about how proteins are modified by glycosylation. For clients looking for thorough protein characterization, Creative Proteomics' expertise in glycosylation analysis, along with our cutting-edge technology and personalized services, offers a distinct advantage. We are in a good position to help our clients with the development of their protein drugs thanks to our knowledge and dedication to quality.
In addition, we have a range of protein drug characterization services, so please feel free to contact us if you are interested in our services or have any other questions. We look forward to working with you on your attractive projects.
References
- Zhang, S., et al.; Quantification of N-glycosylation site occupancy status based on labeling/label-free strategies with LC-MS/MS. Talanta. 2017, 170, 509–513.
- Zhu, Z., et al.; Absolute Quantitation of Glycosylation Site Occupancy Using Isotopically Labeled Standards and LC-MS. Journal of The American Society for Mass Spectrometry. 2014, 25(6).
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang, S., et al.;)
Fig 1. MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang, S., et al.;) Fig 2. Peptide standards with heavy isotope-labeled tryptic termini (denoted as *R/K) are spiked to quantify the total protein concentration and the non-glycosylated protein concentration (Zhu, Z., et al.; 2014)
Fig 2. Peptide standards with heavy isotope-labeled tryptic termini (denoted as *R/K) are spiked to quantify the total protein concentration and the non-glycosylated protein concentration (Zhu, Z., et al.; 2014)

