To meet the requirements of ICH Q6B guidelines, Creative Proteomics offers services to analyze the tertiary structure of proteins, which can precisely understand the morphology, size, shape, interaction sites, and mechanism of interaction with other molecules of proteins. These services also reveal the relationship between the structure and function of proteins.
What is Protein Tertiary Structure?
Protein tertiary structure refers to the three-dimensional arrangement of atoms in a single protein molecule, including the spatial arrangement of secondary structural elements, as well as loops and other irregular regions. It is determined by the interactions between amino acid residues that are nearby in the linear sequence of the protein.
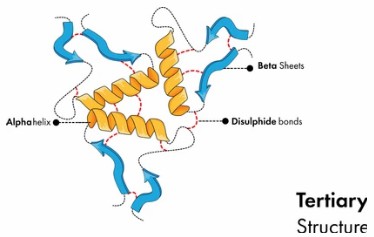 Fig 1. Protein Tertiary Structure.
Fig 1. Protein Tertiary Structure.
The tertiary configuration of a protein greatly influences its functionality by delineating the way in which disparate domains converge to establish a functional entity, such as an active locus for enzyme catalysis or a ligand recognition site. Interplays that contribute to this tertiary architecture encompass hydrogen and disulfide bonding, hydrophobic and electrostatic interplay, in addition to van der Waals forces.
How to Detect Protein Tertiary Structure?
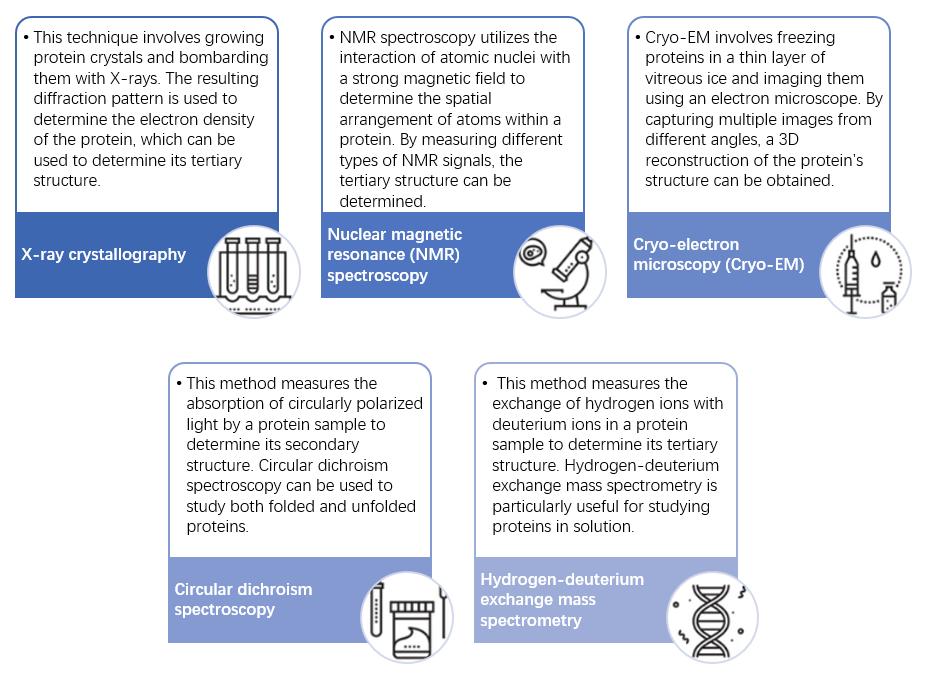 Fig 2. Methods for detecting the tertiary structure of proteins.
Fig 2. Methods for detecting the tertiary structure of proteins.
Challenges of Tertiary Structure Analysis
Structural Complexity
Structural complexity of proteins is significantly high, characterized by an in-depth array of secondary structure determinants including alpha helices and beta sheets, not excluding loops and turns. Ascertainment of the exact molecular organization of these structures represents a considerable challenge, especially with respect to proteins of considerable size and those possessing multiple domains.
Experimental Techniques
A plethora of experimental methodologies exist for the examination of protein tertiary structures, ranging from X-ray crystallography to nuclear magnetic resonance (NMR) spectroscopy and cryo-electron microscopy (cryo-EM). Each methodology encompasses its unique set of limitations and requisites, underscoring the necessity of methodical selection in accordance with the specific characteristics of the protein under study.
Sample Preparation
The procurement of superior quality protein prototypes, embodying the precise conformation, is an indispensable step for executing an unerring structural scrutiny. Nevertheless, an array of proteins might prove to be formidable in terms of production at an extensive scale or procuring the anticipated conformation necessary for analysis. The stability, purification, and solubility of proteins are key variables that demand meticulous attention during the convoluted process of sample formulation.
Data Interpretation
The interpretation of data derived from structural analysis techniques necessitates meticulous scrutiny, particularly in the context of protein structure determination, which frequently employs multifaceted computational algorithms and modeling methodologies to accurately align with experimental findings. The precision and dependability of the interpreted data are of paramount importance in deducing significant conclusions.
Dynamic Nature
Proteins do not exist as unchanging units. They possess the ability to undergo structural modifications and exhibit dynamism, both features being integral to their function. Capturing these dynamic characteristics of proteins in their native environment, while posing a significant challenge, is indispensable for an in-depth comprehension of their structure-function nexus.
Our Service for Tertiary Structure Analysis
- Knowing the structure and function of proteins
- Directing protein modification and design
- Predicting proteins' dynamic characteristics
- Understanding protein interactions
Our Advantages

Decades of Experience
With over 20 years of experience in the field, Creative Proteomics has amassed a wealth of knowledge and expertise, allowing us to deliver top-notch results.

Cutting-Edge Facilities
Our company houses state-of-the-art laboratories equipped with the latest instrumentation and software, ensuring the highest level of precision and accuracy.

Interdisciplinary Team
Our team consists of highly skilled scientists from diverse disciplines, including biochemistry, biophysics, and computational biology.
Our Goals
Protein tertiary structure analysis plays a crucial role in decoding the complex 3D world of proteins. With various experimental and computational methods at our disposal, Creative Proteomics offers unparalleled services in this domain. Our commitment to excellence, state-of-the-art facilities, and interdisciplinary team ensure that we remain at the forefront of protein structure analysis, enabling advancements in several fields of science and medicine. Please feel free to contact us if you have any specific requests regarding our service for determining protein sequences.
Reference
- Rico, F., et al.; Mechanics of proteins with a focus on atomic force microscopy. Journal of Nanobiotechnology. 2013, 11(Suppl 1), S3.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Protein Tertiary Structure.
Fig 1. Protein Tertiary Structure. Fig 2. Methods for detecting the tertiary structure of proteins.
Fig 2. Methods for detecting the tertiary structure of proteins.







