Creative Proteomics provides higher-order structure analysis of proteins, which can help customers gain a deeper understanding of protein functions and biological processes, providing important support for the development of life sciences. It also meets the requirements of ICH Q6B guidelines for structural characterization.
What is Protein Higher-Order Structure?
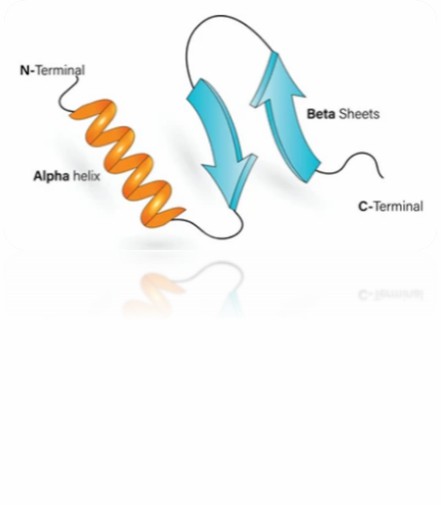
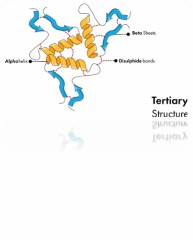
Protein higher-order structure delineates the overarching configuration and disposition of protein sub-domains or individual structural components. This concept pertains to the tri-dimensional spatial arrangement of constituent protein domains or subunits nested within a larger protein assemblage.
Protein higher-order structure is a crucial determinant of their functional adequacy and stability. Proteins can manifest an array of higher-order structure variants ranging from oligomeric conformations (denoting the convergence of multiple subunits), to multimeric configurations (referring to the assembly of diverse protein complexes), even extending to supramolecular arrangements (involving assemblies constituted by the interaction of numerous proteins or macro molecules).
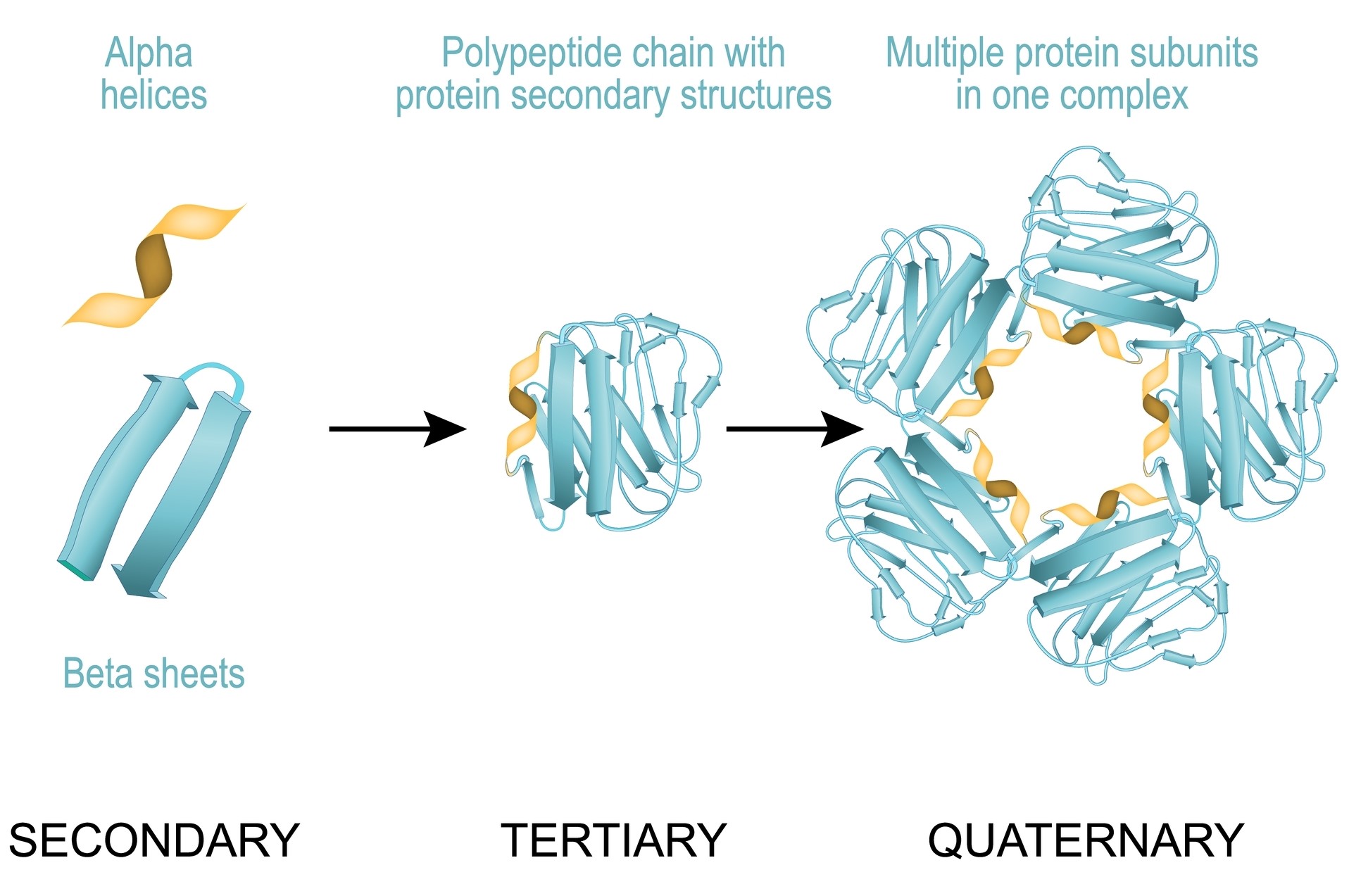 Fig 1. Protein higher-order structure.
Fig 1. Protein higher-order structure.
Protein Higher-Order Structure Techniques
1. X-ray crystallography
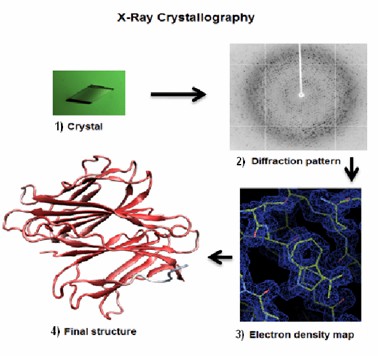 Fig 2. Four main steps to solve a protein structure by X-ray crystallography: (1) to crystallize the protein, (2) to collect the diffraction, (3) to calculate the electron density map, (4) to refine and validate the model of the structure of the protein. (Kumar, S., et al.; 2012)
Fig 2. Four main steps to solve a protein structure by X-ray crystallography: (1) to crystallize the protein, (2) to collect the diffraction, (3) to calculate the electron density map, (4) to refine and validate the model of the structure of the protein. (Kumar, S., et al.; 2012)
X-ray crystallography is an invaluable tool used in elucidating the three-dimensional structures of proteins at atomic resolution. The process primarily involves the growth of high-quality protein crystals, followed by interrogation of these crystals with a set of X-rays. The diffraction pattern resulting from X-ray scattering provides an intricate depiction of the spatial arrangement of atoms within the protein molecule.
2. Nuclear Magnetic Resonance (NMR) spectroscopy
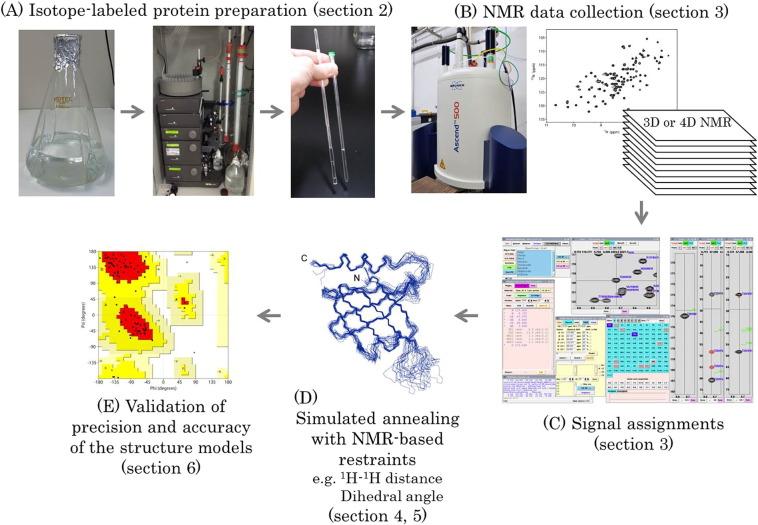 Fig 3. Workflow of protein structure determination by solution NMR spectroscopy. (Sugiki, T., et al.; 2017)
Fig 3. Workflow of protein structure determination by solution NMR spectroscopy. (Sugiki, T., et al.; 2017)
NMR spectroscopy lends itself to the determination of protein structures in their solution states. By probing the spin states of atomic nuclei in a protein under the influence of an external magnetic field, comprehensive data on atomic distances and relative atomic orientations are acquired, facilitating accurate protein structure determination.
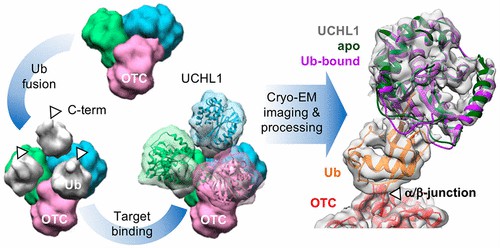 Fig 4. Direct Visualization of a 26 kDa Protein by Cryo-Electron Microscopy Aided by a Small Scaffold Protein. (Chiu, et al.; 2021)
Fig 4. Direct Visualization of a 26 kDa Protein by Cryo-Electron Microscopy Aided by a Small Scaffold Protein. (Chiu, et al.; 2021)
Cryo-EM is a structural biology technique that maintains proteins in a close-to-native state by rapid freezing, followed by examination under high-resolution electron microscopy.
4. Circular Dichroism (CD) spectroscopy
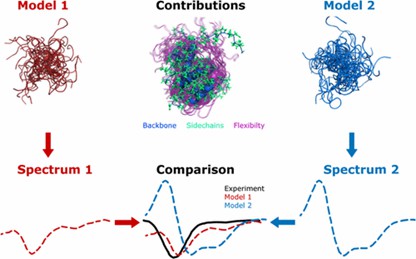 Fig 5. Predicting Circular Dichroism Spectra from Protein Molecular Structures. (Nagy, G., et al.; 2019)
Fig 5. Predicting Circular Dichroism Spectra from Protein Molecular Structures. (Nagy, G., et al.; 2019)
CD spectroscopy is a highly potent technique used predominantly for the analysis of protein secondary structure. This method is based on the dissimilar absorption of left and right-handed circularly polarized light by a protein, allowing for robust interrogation of secondary structure elements like alpha-helices and beta-sheets.
5. Fourier Transform Infrared Spectroscopy (FTIR)
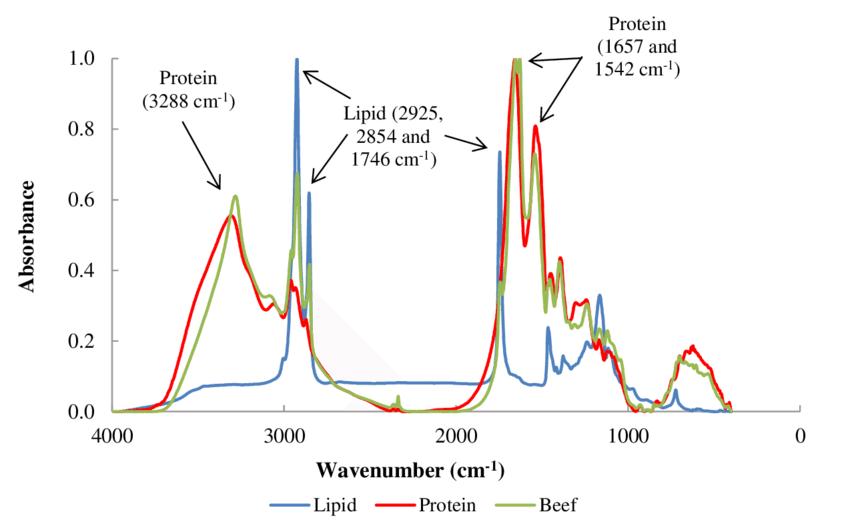 Fig 6. Normalized lipid, protein and meat (beef) FTIR spectra. (Lozano, M., et al.; 2017)
Fig 6. Normalized lipid, protein and meat (beef) FTIR spectra. (Lozano, M., et al.; 2017)
FTIR spectroscopy is another powerful technique used to discern protein secondary structure by measuring the absorption of infrared light by a protein. This gives comprehensive structural detail about alpha helices, beta-sheets, and other secondary structural features such as random coils.
6. Small-Angle X-ray Scattering (SAXS)
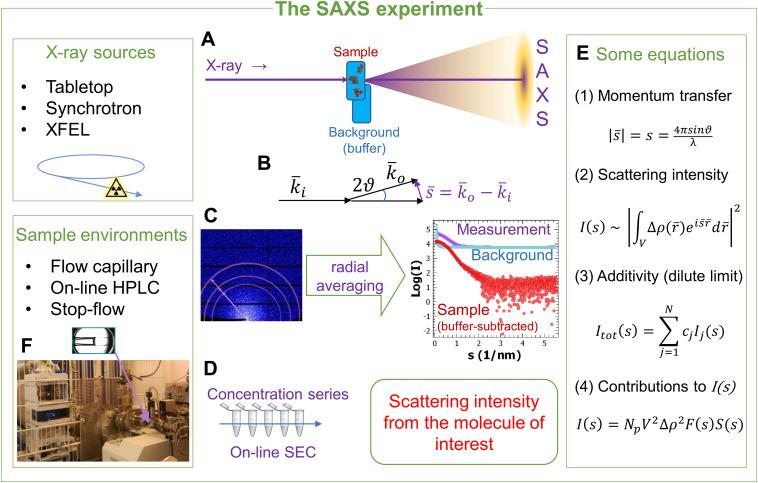 Fig 7. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. (Da Vela, S., & Svergun, D. I.; 2020)
Fig 7. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. (Da Vela, S., & Svergun, D. I.; 2020)
SAXS technique enables the determination of protein structure in solution, providing key insights about shape, size, and conformation, and giving a broad overview of the protein's higher-order structure.
7. Hydrogen-Deuterium Exchange Mass Spectrometry (HDX-MS)
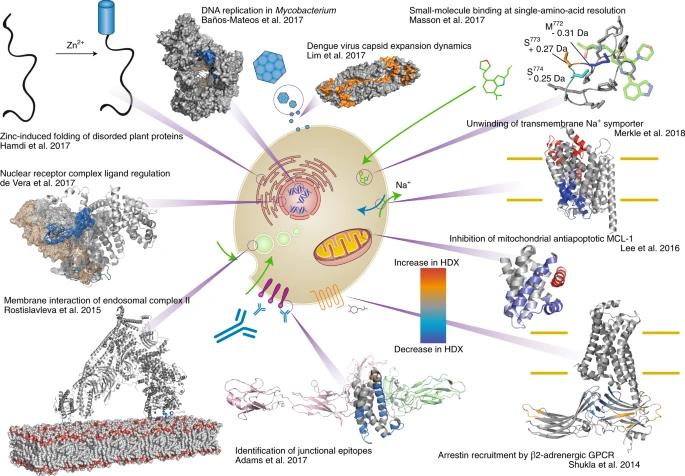 Fig 8. The wide range of applications for HDX-MS in many protein-folding studies. (Masson, et al.; 2019)
Fig 8. The wide range of applications for HDX-MS in many protein-folding studies. (Masson, et al.; 2019)
HDX-MS is a technique that examines the dynamics of hydrogen-deuterium exchange in proteins. By characterizing the rate of this exchange, detailed insights into the intricate processes of protein folding, conformational dynamics, and higher-order structures are achieved.
Significance for Protein Higher-Order Structure Analysis
Understanding the higher-order structure of proteins helps to understand the biological functions of proteins, because higher-order structure is closely related to the biological activity of proteins. For example, the secondary structure of a protein can affect its interaction with other molecules, while the tertiary structure can influence the active state and stability of the protein.
The study of protein advanced structure also helps to reveal the relationship between protein structure and function, thus providing an important theoretical basis for protein design, modification and synthesis. For example, by understanding the higher-order structure of proteins, it is possible to predict the behavior of proteins in specific environments, such as inside or outside the cell or at interfaces.
Analyzing the higher-order structure of proteins can also help us understand the relationship between the structure of proteins and the occurrence, development and treatment of diseases, thus providing new ideas and methods for drug design and disease treatment. For example, by understanding the higher-order structure of proteins, specific drug molecules can be designed to bind to proteins and affect their functions, thereby treating the relevant diseases.
Our Services
To help researchers better understand the structure and characteristics of proteins, Creative Proteomics offers protein secondary structure analysis services. These analyses look at the spatial orientation of protein peptide chains along the main chain backbone direction, the regular cyclic arrangement, or the local spatial structure of a section of peptide chain. This information is then used to inform drug design, biological therapy, and other areas.
To assist our clients in better understanding the biological functions of proteins and their relationship to the occurrence and progression of diseases, Creative Proteomics offers tertiary structure analysis of proteins, which examines the three-dimensional spatial structure of the entire protein molecule, including the relative positions, orientations, and linkages of all amino acid residues. the connection. This aids in the creation of novel treatments and medications as well as the design and synthesis of proteins with particular roles.
Our Goals
At Creative Proteomics, our ultimate goal is to contribute to scientific advancements by providing innovative solutions for protein high-order structure analysis. We strive to exceed our clients' expectations and establish long-term partnerships within the scientific community. Through our services, we aim to unravel the mysteries of protein primary structure and facilitate breakthrough discoveries in various fields, including medicine, agriculture, and biotechnology. If you have any special requirements about our protein sequence determination service, please feel free to contact us.
References
- Kumar, S., et al.; In Silico Engineering of Proteins That Recognize Small Molecules. Protein Engineering. 2012.
- Sugiki, T., et al.; Modern Technologies of Solution Nuclear Magnetic Resonance Spectroscopy for Three-dimensional Structure Determination of Proteins Open Avenues for Life Scientists. Computational and Structural Biotechnology Journal. 2017, 15, 328–339.
- Chiu, et al.; Direct Visualization of a 26 kDa Protein by Cryo-Electron Microscopy Aided by a Small Scaffold Protein. Biochemistry. 2021, 60(14), 1075–1079.
- Nagy, G., et al.; SESCA: Predicting Circular Dichroism Spectra from Protein Molecular Structures. Journal of Chemical Theory and Computation. 2019.
- Lozano, M., et al.; Mid-Infrared Spectroscopy (MIR) for Simultaneous Determination of Fat and Protein Content in Meat of Several Animal Species. Food Analytical Methods. 2017, 10(10), 3462–3470.
- Da Vela, S., & Svergun, D. I. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. Current Research in Structural Biology. 2020, 2, 164–170.
- Masson, et al.; Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nature Methods. 2019, 16(7), 595–602.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Protein higher-order structure.
Fig 1. Protein higher-order structure. Fig 2. Four main steps to solve a protein structure by X-ray crystallography: (1) to crystallize the protein, (2) to collect the diffraction, (3) to calculate the electron density map, (4) to refine and validate the model of the structure of the protein. (Kumar, S., et al.; 2012)
Fig 2. Four main steps to solve a protein structure by X-ray crystallography: (1) to crystallize the protein, (2) to collect the diffraction, (3) to calculate the electron density map, (4) to refine and validate the model of the structure of the protein. (Kumar, S., et al.; 2012) Fig 3. Workflow of protein structure determination by solution NMR spectroscopy. (Sugiki, T., et al.; 2017)
Fig 3. Workflow of protein structure determination by solution NMR spectroscopy. (Sugiki, T., et al.; 2017) Fig 4. Direct Visualization of a 26 kDa Protein by Cryo-Electron Microscopy Aided by a Small Scaffold Protein. (Chiu, et al.; 2021)
Fig 4. Direct Visualization of a 26 kDa Protein by Cryo-Electron Microscopy Aided by a Small Scaffold Protein. (Chiu, et al.; 2021) Fig 5. Predicting Circular Dichroism Spectra from Protein Molecular Structures. (Nagy, G., et al.; 2019)
Fig 5. Predicting Circular Dichroism Spectra from Protein Molecular Structures. (Nagy, G., et al.; 2019) Fig 6. Normalized lipid, protein and meat (beef) FTIR spectra. (Lozano, M., et al.; 2017)
Fig 6. Normalized lipid, protein and meat (beef) FTIR spectra. (Lozano, M., et al.; 2017) Fig 7. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. (Da Vela, S., & Svergun, D. I.; 2020)
Fig 7. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. (Da Vela, S., & Svergun, D. I.; 2020) Fig 8. The wide range of applications for HDX-MS in many protein-folding studies. (Masson, et al.; 2019)
Fig 8. The wide range of applications for HDX-MS in many protein-folding studies. (Masson, et al.; 2019)