Protein oxidation is linked to a variety of life activities, including aging and disease onset. Protein oxidation analysis aids in understanding the role and modifications of proteins in these processes. Creative Proteomics offers complete protein oxidation analysis services that adhere to the ICH Q6B guidelines.
Background
Protein oxidation refers to the process of reactive oxygen species (ROS) or other reactive molecules negatively affecting the structure and function of proteins within a living system. ROS are highly reactive molecules that are produced as byproducts of normal cellular metabolism or in response to environmental stressors like radiation, pollution, or toxins.
When proteins are oxidized, their amino acid residues may undergo modifications, such as oxidation of methionine, cysteine, or tryptophan, as well as protein cross-linking or fragmentation. These modifications can disrupt the structural integrity of proteins, impair their biological activity, or lead to aggregation and misfolding.
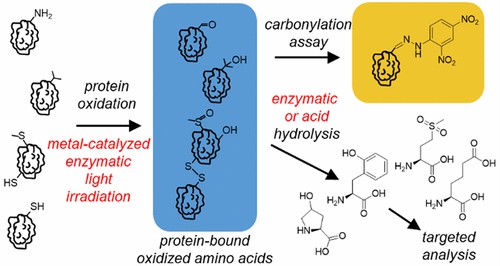 Fig 1. Analysis of Protein Oxidation in Food and Feed Products. (Hellwig, M.; 2020)
Fig 1. Analysis of Protein Oxidation in Food and Feed Products. (Hellwig, M.; 2020)
Oxidative Protein Modification Mechanisms
Oxidation of sulfur-containing amino acids
The phenomenon under consideration pertains to the oxidative modification of sulfur-enriched amino acids such as cysteine and methionine. This transpires as the result of oxidative sequences leading to the production of sulfite, sulfurous acid, and diverse sulfonic acid derivatives. We find that amino acids integrating sulfur display particular susceptibility to oxidative stress. Modifications of this oxidative nature have profound implications on both the architecture and functionality of proteins.
Oxidation of aromatic moieties
Aromatic molecules, including tyrosine and phenylalanine, undergo oxidative alterations. The oxidation of aromatic rings can lead to the formation of transient intermediates such as semiquinones and quinones. These processes are exceptionally catalyzed by Reactive Oxygen Species (ROS). Moreover, these reactive species have the potential to inflict oxidative harm by engaging with other biomolecular entities.
Glycoxidation
Glycoxidation represents a biochemical process where reactive carbonyl entities, predominantly sugars like glucose and fructose, induce modifications in proteins in the context of oxidative stress. This molecular mechanism ultimately results in the production of end materials known as Advanced Glycation End products (AGEs). Research has linked glycoxidation to various pathologies, including but not limited to diabetes and neurodegenerative diseases, owing to its potential to alter both the structural and functional properties of proteins.
Lipoxidation
Reactive oxygen species alter lipids (such as polyunsaturated fatty acids) by oxidation. Lipid peroxides are created during this process, and they later interact with proteins to create adducts. Lipid oxidation can alter proteins' and lipids' structural and functional properties, which can result in cellular malfunction and tissue damage.
Carbonylation
Carbonyl groups are added to proteins during carbonylation. Different mechanisms, such as the interaction of reactive oxygen species or reactive carbonyl compounds, can cause it to happen. Protein malfunction and aggregation caused by carbonylation can hinder cellular function. Proteins that have been carbonylated are thought to be signs of oxidative damage and aging.
Nitration/nitrosylation
Adding a nitro group (-NO2) to a protein is known as nitrosylation, whereas adding a nitric oxide (NO) group is known as nitrosylation. Reactive nitrogen reacts with the protein to produce both processes. Protein structure and function can be changed by nitrosylation and nitroration, which can have an impact on signaling pathways and physiological activities.
Protein Oxidation Analysis Methodology
1.Western blotting
The Western blotting methodology facilitates the identification of oxidized proteins through the deployment of particular antibodies that detect oxidation-specific epitopes. This approach offers both qualitative and semi-quantitative insights into the magnitude of protein oxidation.
2.Mass spectrometry
Mass spectrometry represents a potent analytical modality, capable of discerning and quantifying particular oxidized amino acids present within proteins. Its operational precision enables the exact determination of the locational and categorical facets of oxidation transpiring within protein structures.
3.Carbonyl assay
Protein carbonylation, a general oxidative modification that occurs biologically, is identifiable utilizing the carbonyl assay methodology. The process of implementing this assay comprises the derivatization of carbonyl groups in proteins by using a chemical substance known as 2,4-dinitrophenylhydrazine (DNPH). Upon derivatization, detection is accomplished either spectrophotometrically or through the application of immunoblot techniques.
4.Fluorescent probes
It is noteworthy to illuminate that multiple fluorescent probes, specifically 2,7-dichlorofluorescein (DCFH) and dihydroethidium (DHE), can exhibit efficacy in detecting the production of reactive oxygen species (ROS) and subsequent oxidative stress manifested in various cells and tissues. The employed probes also serve as indirect indicators of oxidation occurrence in proteinaceous materials.
5.Redox-sensitive dyes
The redox status of protein thiols can be ascertained through the utilization of specific dyes, notably 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) and 4-chloro-7-nitrobenzofurazan (NBD-Cl). Analyzing the oxidation or reduction processes of these dyes provides valuable insights into protein oxidation.
6.Protein fragmentation assays
Protein oxidation can trigger fragmentation, which subsequently releases proteolytic fragments. Employing analytical methodologies such as gel electrophoresis and mass spectrometry can aid in the identification and quantification of these fragments. This can potentially provide critical insight into the process of protein oxidation, highlighting its molecular implications.
Service Process

Identify Problem

Discussion

Testing

Reporting
Want to Learn More?
At Creative Proteomics, we specialize in offering the pharmaceutical sector trustworthy protein oxidation analysis services. We are dedicated to assuring the stability and effectiveness of your pharmaceutical products, and we have years of experience in protein drug characterization. Please get in touch with us if you have any questions about our services for protein oxidation analysis.
Reference
- Hellwig, M. Analysis of Protein Oxidation in Food and Feed Products. Journal of Agricultural and Food Chemistry. 2020, 68, 46, 12870–12885
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Analysis of Protein Oxidation in Food and Feed Products. (Hellwig, M.; 2020)
Fig 1. Analysis of Protein Oxidation in Food and Feed Products. (Hellwig, M.; 2020)