Introduction to Basic Fibroblast Growth Factor
Basic fibroblast growth factor (bFGF) is a recombinant, human, single-chain, nonglycosylated polypeptide comprised of 154 amino acids and possessing a molecular mass in the range of 18 KDa (17,123 Dalton, as confirmed by mass spectrometry readings). This specific polypeptide assumes a pivotal role as a primary inducer in the formation of mesoderm during embryogenesis while also notably influencing cell proliferation and differentiation both in vitro and in vivo settings.
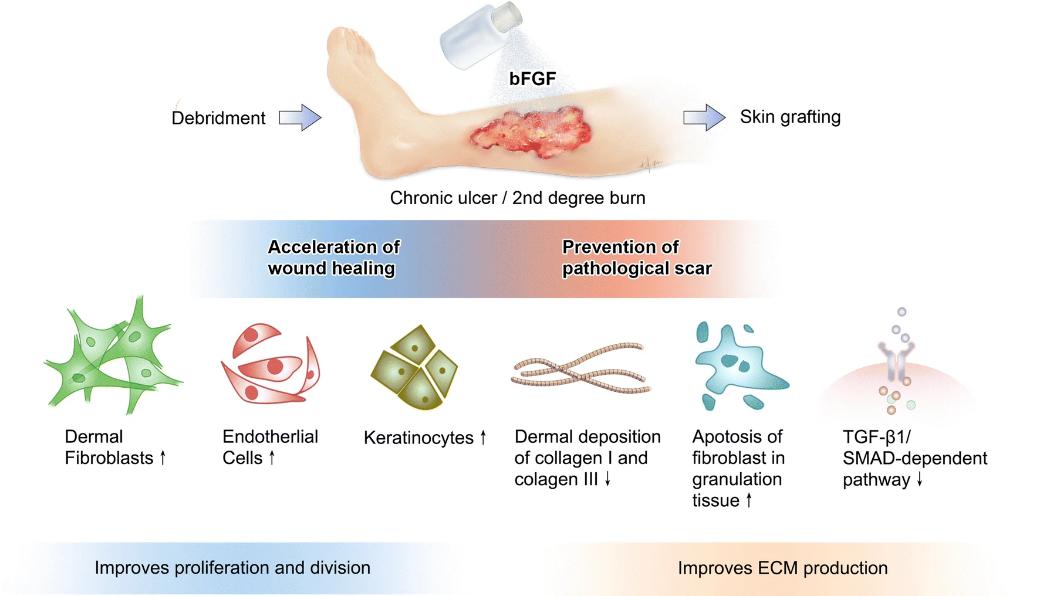 Fig 1. Illustrative overview of the biological actions of bFGF application in chronic ulcer and burn injury involved in accelerating wound healing and preventing pathological scar development. (Abdelhakim, et al.; 2020)
Fig 1. Illustrative overview of the biological actions of bFGF application in chronic ulcer and burn injury involved in accelerating wound healing and preventing pathological scar development. (Abdelhakim, et al.; 2020)
Elucidating further on its numerous functionalities, bFGF stands as a mitogen and chemoattractant, giving it an integral role in processes such as wound healing, angiogenesis, and neural outgrowth. It bears significant potential for clinical applications, chiefly with respects to wound healing, collateral blood vessel formation following myocardial infarcts, and osteogenesis associated with bone fractures. The therapeutic proteins residing in these factories that produce bFGF are presently under rigorous analysis to ascertain their efficacy.
Structure and Conformation
Crystals of bFGF, a protein consisting of 146 amino acid residues, were formulated via the hanging drop vapor diffusion technique employing a solution mixture of ammonium sulfate, citrate or Tris, and the reducing agent, dithiothreitol. X-ray diffraction was implemented to discern the structure, acquiring a resolution between 1.6 and 2.5 Å. As a fundamental component of the protein's molecular architecture, bFGF exhibits a β-barrel backbone structure composed of 12 antiparallel β strands interconnected by βturns, thereby exhibiting an approximate threefold axis of symmetry. This globular protein, with a diameter of around 4 nm, posses hydrophobic residues at its core, while numerous charged residues are distributed over its surface.
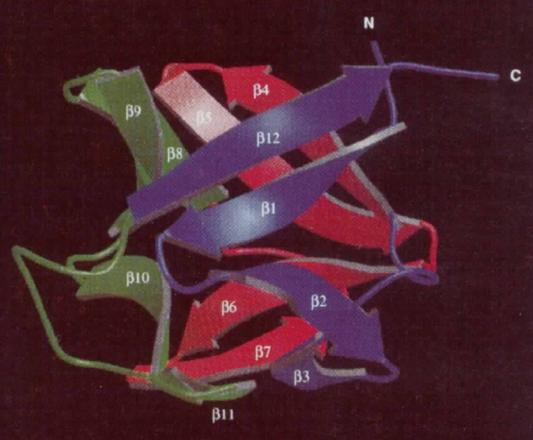 Fig 2. Schematic picture of the fl-trefoil fold of hbFGF 154 rotated 90°. (Kastrup, J. S., et al. 1997)
Fig 2. Schematic picture of the fl-trefoil fold of hbFGF 154 rotated 90°. (Kastrup, J. S., et al. 1997)
Thermal Stability as a Function of pH
The pH dependence of thermal denaturation of bFGF was studied in a phosphatecitrate–borate buffer system. The endothermic shift prominently begins to display above pH 4. Remarkably, an elevation was noticed in the temperature range, shifting from an initial 50°C to 64°C, as the pH values alternated between 4 and 9. This temperature change appeared to stabilize and reach a plateau when a pH value of between 7 and 9 was maintained. These findings inferred that the structural conformation of bFGF enters into a state of optimal thermal stability within the pH window of 7 to 9.
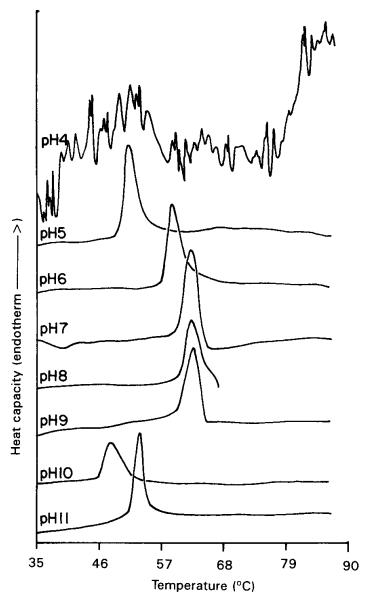 Fig 3. pH-Dependent change in DSC endothermic peak of bFGF. (Vemuri, Sriram, et al. 1994)
Fig 3. pH-Dependent change in DSC endothermic peak of bFGF. (Vemuri, Sriram, et al. 1994)
Sites of Cysteine Oxidation
The cysteine residues situated at the 34, 78, 96, and 101 positions within bFGF can potentially engage in intramolecular or intermolecular disulfide formations. Utilizing site-directed mutagenesis allows us to substitute cysteines with serines, leading to the creation of 15 different muteins (four muteins from four serine replacements, one from three replacements, six from two replacements, and four from a single serine substitution).
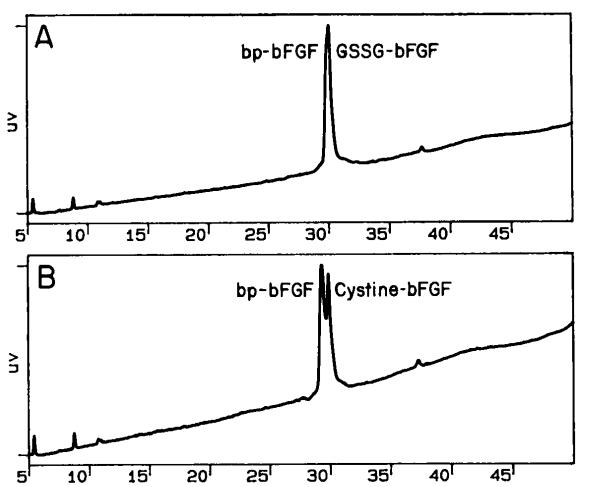 Fig 4. Reversed-phase chromatography of bovine pituitary bFGF and S-thiolated forms of recombinant bFGF. (Thompson, S. A., et al. 1991)
Fig 4. Reversed-phase chromatography of bovine pituitary bFGF and S-thiolated forms of recombinant bFGF. (Thompson, S. A., et al. 1991)
Insights derived from these muteins' performance on heparin affinity High-Performance Liquid Chromatography (HPLC) highlight that the most reactive cysteines were present at the 78 and 96 positions. By contrast, the cysteines at the 34 and 101 positions demonstrated lower reactivity, which might be ascribed to the constraints imposed by their conformational structuring or an intramolecular disulfide bond formation.
What Can We Offer?
Creative Proteomics stands as a distinguished entity within the proteomics industry, priding ourselves as purveyors of high-quality protein drug characterization services. Our proficiency extends to serving a diverse array of clientele comprising pharmaceutical establishments, research organizations, and esteemed academics. We welcome further inquiries, kindly do not hesitate to initiate contact.
References
- Abdelhakim, et al. The Japanese experience with basic fibroblast growth factor in cutaneous wound management and scar prevention: a systematic review of clinical and biological aspects. Dermatology and therapy. 2020, 10: 569-587.
- Kastrup, J. S., et al. X-ray Structure of the 154-Amino-Acid Form of Recombinant Human Basic Fibroblast Growth Factor. Comparison with the Truncated 146-Amino-Acid Form. Acta Crystallographica Section D Biological Crystallography. 1997, 53(2), 160–168.
- Vemuri, Sriram, et al. The stability of bFGF against thermal denaturation. Journal of pharmacy and pharmacology. 1994, 46.6: 481-486.
- Thompson, S. A., et al. Chemical characterization of the cysteines of basic fibroblast growth factor. Annals of the New York Academy of Sciences. 1991, 638.1: 78-88.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. Illustrative overview of the biological actions of bFGF application in chronic ulcer and burn injury involved in accelerating wound healing and preventing pathological scar development. (Abdelhakim, et al.; 2020)
Fig 1. Illustrative overview of the biological actions of bFGF application in chronic ulcer and burn injury involved in accelerating wound healing and preventing pathological scar development. (Abdelhakim, et al.; 2020) Fig 2. Schematic picture of the fl-trefoil fold of hbFGF 154 rotated 90°. (Kastrup, J. S., et al. 1997)
Fig 2. Schematic picture of the fl-trefoil fold of hbFGF 154 rotated 90°. (Kastrup, J. S., et al. 1997) Fig 3. pH-Dependent change in DSC endothermic peak of bFGF. (Vemuri, Sriram, et al. 1994)
Fig 3. pH-Dependent change in DSC endothermic peak of bFGF. (Vemuri, Sriram, et al. 1994) Fig 4. Reversed-phase chromatography of bovine pituitary bFGF and S-thiolated forms of recombinant bFGF. (Thompson, S. A., et al. 1991)
Fig 4. Reversed-phase chromatography of bovine pituitary bFGF and S-thiolated forms of recombinant bFGF. (Thompson, S. A., et al. 1991)