Introduction to Transforming Growth Factor-β1 (TGF-β1)
The transforming growth factor-beta (TGF-β) family of proteins has emerged as a particularly noteworthy group due to its wide-ranging multifunctional capabilities. Notably, one member of this family, namely transforming growth factor-beta1 (TGF-β1), was initially identified for its ability to induce a phenotypic transformation in rat fibroblasts.
 Fig 1. Architecture of proTGF-β1. (Shi, Minlong, et al. 2011)
Fig 1. Architecture of proTGF-β1. (Shi, Minlong, et al. 2011)
Since its discovery, TGF-β1 has been found to exert significant regulatory actions on both normal and neoplastic cells. Importantly, nearly all cell types possess specific high-affinity receptors for TGF-β, and multiple cell types are capable of synthesizing this protein. Consequently, TGF-β stands as a pivotal regulatory molecule that can elicit its effects through autocrine and paracrine mechanisms.
Structure
Mature TGF-β1 is composed of two identical peptide chains, each consisting of 112 amino acids. The protein is a homodimer with a molecular weight of 24 kDa and contains a total of nine disulfide bonds. One of these bonds links the monomeric subunits together. When reduced, the homodimer dissociates into two identical peptides, each weighing approximately 12 kDa.
The nonreduced form of the homodimer is the biologically active form of the molecule. Although the crystal structure of TGF-β1 is currently unknown, it has been determined for TGF-β2. TGF-β1 shares 71% homology with TGF-β2 in terms of amino acid sequence, indicating a probable structural similarity between the two proteins.
 Fig 2. A Sequence comparisons and 3-D-1-D profile scores for the TGF-β superfamily. (Daopin, S., et al. 1992)
Fig 2. A Sequence comparisons and 3-D-1-D profile scores for the TGF-β superfamily. (Daopin, S., et al. 1992)
Studies on TGF-β2 have shown that it lacks a well-defined hydrophobic core and instead exhibits an unusual elongated nonglobular fold. This fold has dimensions of approximately 60 Å by 20 Å by 15 Å. Sequence analysis of other members of the TGF-β superfamily suggests that they also adopt this unique fold.
Within the TGF-β1 protein, eight cysteines form four intrachain disulfide bonds, which are localized in a central core region. The dimerization of the protein is further stabilized by the ninth cysteine, which forms an interchain disulfide bond. Additionally, there are two identical hydrophobic interfaces that contribute to the stability of the dimeric structure.
Integrity of TGF-β1
RP-HPLC (Reverse Phase High Performance Liquid Chromatography) methodology is utilized to scrutinize the integrity of TGF-β1 by distinguishing TGF-β1 and its derivatives, grounded on the discrepancy in their partitioning among the hydrophobic matrix within the column and the comparatively hydrophilic mobile phase.
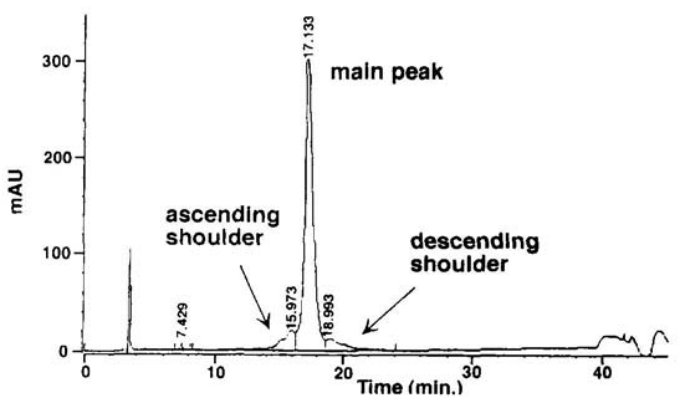 Fig 3. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) of TGF-β1. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) of TGF-β1. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
What Can We Offer?
As a highly experienced professional in the field of biology, coupled with a team of accomplished scientists, we at Creative Proteomics strive to offer precise and dependable analyses. Our portfolio of services grant comprehensive insights into the attributes, properties, and functions of protein drugs. These extend to elements such as protein structure determination, stability evaluation, assessment of post-translational modifications, identification, and quantification of impurities. We welcome your queries.
References
- Shi, Minlong, et al. Latent TGF-β structure and activation. Nature. 2011, 474.7351: 343-349.
- Daopin, S., et al. Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily. Science. 1992, 257(5068), 369–373.
- Pearlman, Rodney, and Y. John Wang, eds. Formulation, characterization, and stability of protein drugs. Vol. 9. Springer Science & Business Media. 1996.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. Architecture of proTGF-β1. (Shi, Minlong, et al. 2011)
Fig 1. Architecture of proTGF-β1. (Shi, Minlong, et al. 2011) Fig 2. A Sequence comparisons and 3-D-1-D profile scores for the TGF-β superfamily. (Daopin, S., et al. 1992)
Fig 2. A Sequence comparisons and 3-D-1-D profile scores for the TGF-β superfamily. (Daopin, S., et al. 1992) Fig 3. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) of TGF-β1. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) of TGF-β1. (Pearlman, Rodney, and Y. John Wang, eds. 1996)