At Creative Proteomics, we perform amino acid analysis (AAA) to determine the composition and quantity of amino acids present in a protein or biological sample. We are committed to providing our customers with automated and highly sensitive analytical methods that comprehensively analyze amino acid sequences, enabling you to meet the biotechnology/biologics acceptance criteria of the ICH Q6B guidelines.
Amino Acid Analysis
Amino acids, as organic constituents, operate as the primary elements in protein formulation. These molecular structures incorporate an amino grouping (-NH2), a carboxyl division (-COOH), and a distinctive auxiliary chain (R group), bringing uniqueness to each amino acid variation. The configuration and sequential order of these amino acids primarily map out the tri-dimensional formation and functionality of proteins, which underscores their universal presence across all life forms.
Analyzing Amino Acids (AAA) is a pivotal tool within the biological realm, facilitating a comprehensive examination of the amino acids existing in a given sample. The resultant data from this careful analysis lends critical information on aspects such as protein content, protein concentration, protein degeneration, amongst other pertinent factors.
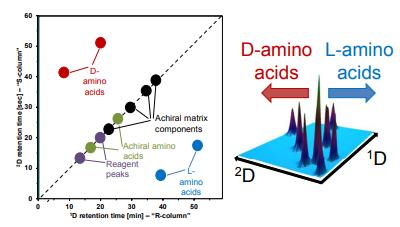 Fig 1. Imaging peptide and protein chirality via amino acid analysis. (Woiwode, U., et al; 2018)
Fig 1. Imaging peptide and protein chirality via amino acid analysis. (Woiwode, U., et al; 2018)
Amino Acid Analysis Methodology
1. Ion Exchange Chromatography
Ion exchange chromatography (IEC) is an advanced method that leverages variances in charge attributes for the discrimination and separation of ionizable molecules. It has high sample throughput and substantial applicability especially in the fractionation of proteins and enzymes. Furthermore, IEC offers significant advantages including cost-effectiveness, high-resolution separation, scalable operations, and potential automation. Consequently, it is widely recognized as an efficient, versatile and prevalently employed technique in the realm of liquid chromatography.
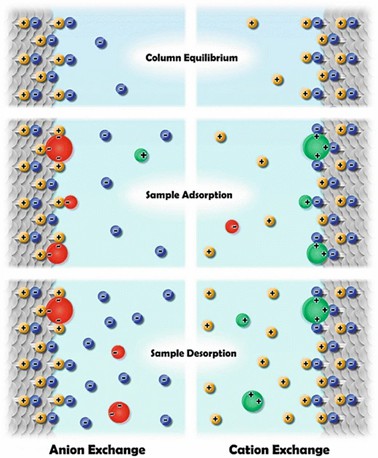 Fig 2. Ion exchange chromatography. (Cummins, P. M., et al.; 2016)
Fig 2. Ion exchange chromatography. (Cummins, P. M., et al.; 2016)
2. Reverse-Phase High-Performance Liquid Chromatography
Reverse-phase high-performance liquid chromatography (RP-HPLC) is the most commonly utilized form of chromatographic study, boasting a vast range of potential uses and a broad selection of mobile and stationary stages. In general, stationary phases consist predominantly of non-polar alkyl hydrocarbons, such as C8 or C18 chains covalently linked to silica or other inert substrates. The labels 'C18’ and ‘C8’ are indicative of the alkyl chain lengths of the column-attached phase.
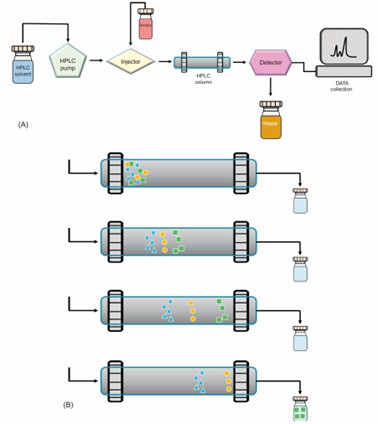 Fig 3. (A) The flow chart of HPLC. (B) The migration of molecules in an HPLC column. (Shen, C.-H. 2019)
Fig 3. (A) The flow chart of HPLC. (B) The migration of molecules in an HPLC column. (Shen, C.-H. 2019)
3. Capillary Electrophoresis
Capillary electrophoresis (CE) is an advanced separation technique deployed to fractionate and analysis charged molecules based on their electrophoretic maneuverability within a capillary. The process involves the electrokinetic movement of charged analytes through an electrolyte-filled capillary under the influence of an applied electric field. The broad-reaching applications of CE include DNA sequencing, protein analysis, drug screening, and environmental study.
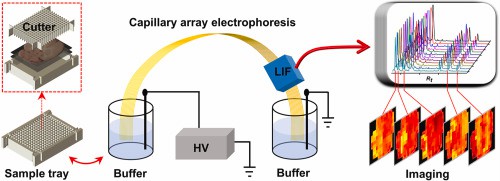 Fig 4. Capillary array electrophoresis imaging of biochemicals in tissue sections. (Qingfeng Z., et al.; 2022)
Fig 4. Capillary array electrophoresis imaging of biochemicals in tissue sections. (Qingfeng Z., et al.; 2022)
4. Mass Spectrometry
Mass spectrometry (MS) is a sophisticated analytical tool employed to elucidate the masses and structural characteristics of molecular entities. The method encompasses the ionization of molecules, followed by their separation based on the mass-to-charge ratio and the subsequent measurement of resulting ion abundance. This allows for the detection, characterization and identification of compounds, including proteins, in intricate mixtures, thereby making it a potent tool in complex biological mixtures' analysis.
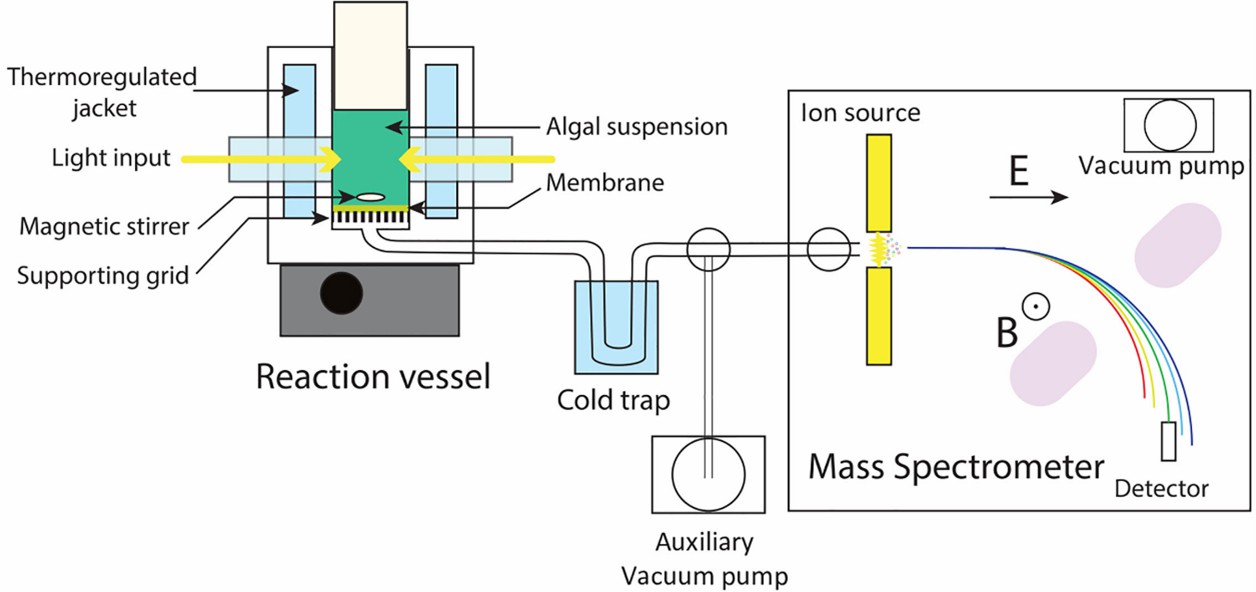 Fig 5. Schematic setup of a Membrane Inlet Mass Spectrometry. (Burlacot, A., et al.; 2020)
Fig 5. Schematic setup of a Membrane Inlet Mass Spectrometry. (Burlacot, A., et al.; 2020)
Amino Acid Analysis Workflow
The methodology for sedimentary amino acid-specific radiocarbon isotope analysis introduced here is subdivided into three main steps: (1) amino acid extraction, (2) amino acid isolation, and (3) final amino acid purification and radiocarbon analysis.
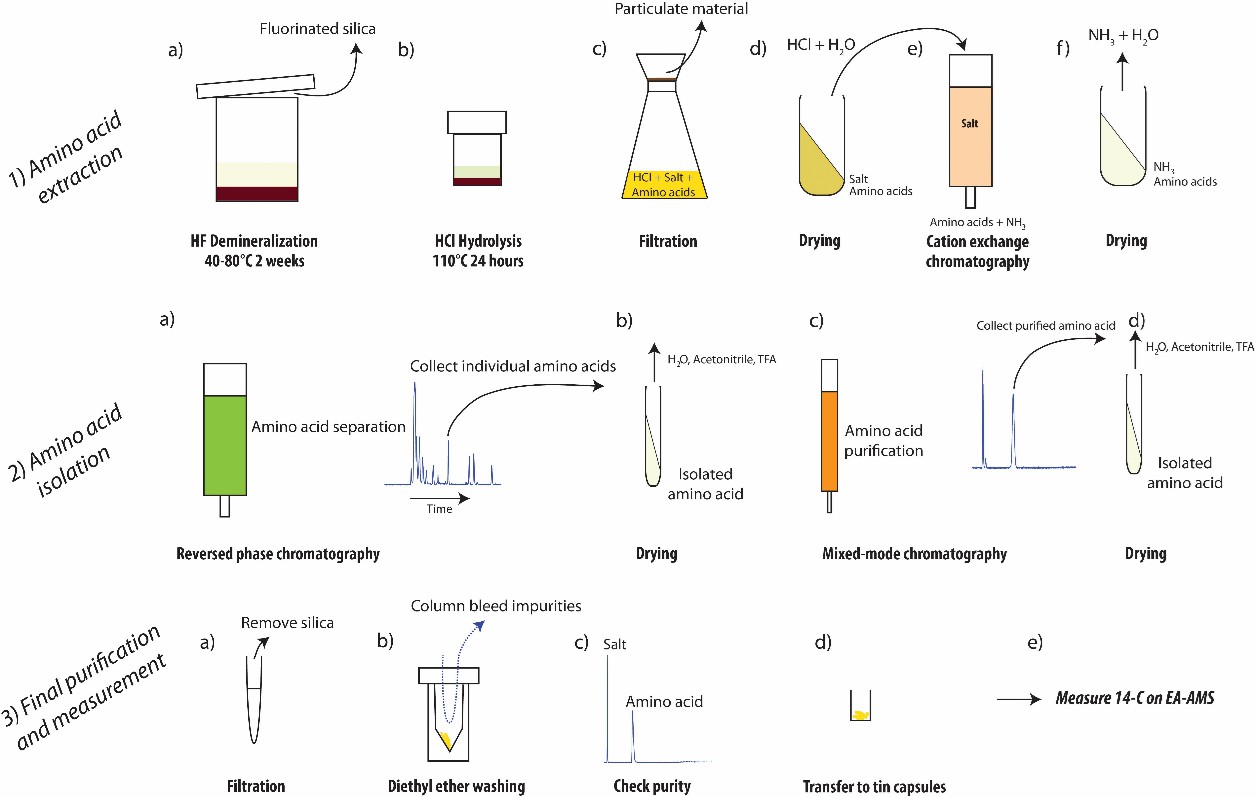 Fig 6. Overview of designed procedure recommended for extraction and preparation of amino acids from sediments for measurement of radiocarbon isotopic composition. (Blattmann, T. M., et al.; 2020)
Fig 6. Overview of designed procedure recommended for extraction and preparation of amino acids from sediments for measurement of radiocarbon isotopic composition. (Blattmann, T. M., et al.; 2020)
Our Services
Our team of adept and erudite scientists diligently employ an array of cutting-edge technologies and methodologies to unveil the myriad intricacies within the realm of comprehensive AAA, meticulously adhering to the zenith of scientific standards.
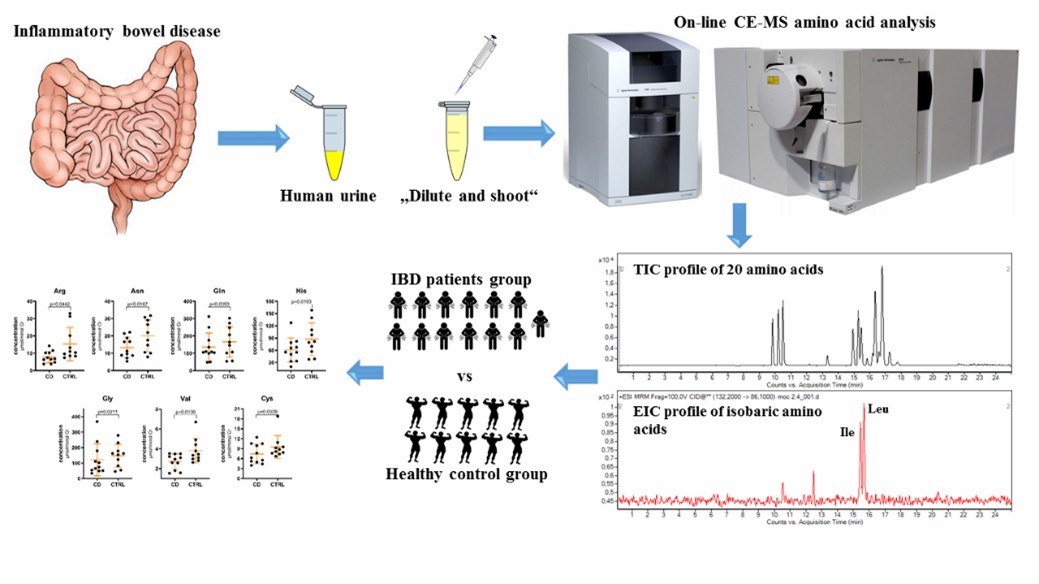 Fig 7. Profiling of Amino Acids in Urine Samples of Patients Suffering from Inflammatory Bowel Disease. (Piestansky, J., et al.; 2019)
Fig 7. Profiling of Amino Acids in Urine Samples of Patients Suffering from Inflammatory Bowel Disease. (Piestansky, J., et al.; 2019)
Sample Preparation
The initiation step in our AAA methodology encompasses rigorous sample preparation. Utilizing cutting-edge methodologies, we extract amino acids from the protein complex, ensuring negligible loss while preserving the integrity of the sample.
Amino Acids Analysis
Following the preparation of the sample, we employ high-performance liquid chromatography (HPLC), integrated with myriad detection techniques, including fluorescence detection and UV detection. This is a method of separating and quantifying the amino acids existing within the sample. The utilization of HPLC offers superior resolution, sensitivity, and reproducibility, rendering our capacity to analyze intricate protein samples with utmost accuracy.
External Standards and Internal Controls
To augment the accuracy of our comprehensive analysis, we implement external standards and internal controls throughout the examination process. The methodology involves contrasting the peak responses from the sample with those of the known standards. Consequently, this allows the precise determination of the identity and concentration of every individual amino acid present.
Our Goals
By providing high-quality and cost-effective amino acid analysis services, Creative Proteomics aims to facilitate research in diverse areas such as drug discovery, protein engineering, and biomarker identification. Through our commitment to excellence, innovation, and customer satisfaction, we are dedicated to being a dependable partner in advancing protein science. If you are interested in our amino acid analysis, please contact us for more details.
References
- Woiwode, U., et al.; Imaging Peptide and Protein Chirality via Amino Acid Analysis by Chiral × Chiral Two-Dimensional Correlation Liquid Chromatography. Analytical Chemistry. 2018, 90(13), 7963–7971.
- Cummins, P. M., et al.; Ion-Exchange Chromatography: Basic Principles and Application. Protein Chromatography. 2016, 209–223.
- Shen, C.-H. Quantification and Analysis of Proteins. Diagnostic Molecular Biology. 2019, 187–214.
- Qingfeng Z, et al.; Capillary array electrophoresis imaging of biochemicals in tissue sections. Talanta. 2022, 240.
- Burlacot, A., et al.; Membrane Inlet Mass Spectrometry: A Powerful Tool for Algal Research. Frontiers in Plant Science. 2020, 11.
- Blattmann, T. M., et al.; Liquid Chromatographic Isolation of Individual Amino Acids Extracted From Sediments for Radiocarbon Analysis. Frontiers in Marine Science. 2020, 7.
- Piestansky, J., et al.; Profiling of Amino Acids in Urine Samples of Patients Suffering from Inflammatory Bowel Disease by Capillary Electrophoresis-Mass Spectrometry. Molecules. 2019, 24(18), 3345.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Imaging peptide and protein chirality via amino acid analysis. (Woiwode, U., et al; 2018)
Fig 1. Imaging peptide and protein chirality via amino acid analysis. (Woiwode, U., et al; 2018) Fig 2. Ion exchange chromatography. (Cummins, P. M., et al.; 2016)
Fig 2. Ion exchange chromatography. (Cummins, P. M., et al.; 2016) Fig 3. (A) The flow chart of HPLC. (B) The migration of molecules in an HPLC column. (Shen, C.-H. 2019)
Fig 3. (A) The flow chart of HPLC. (B) The migration of molecules in an HPLC column. (Shen, C.-H. 2019) Fig 4. Capillary array electrophoresis imaging of biochemicals in tissue sections. (Qingfeng Z., et al.; 2022)
Fig 4. Capillary array electrophoresis imaging of biochemicals in tissue sections. (Qingfeng Z., et al.; 2022) Fig 5. Schematic setup of a Membrane Inlet Mass Spectrometry. (Burlacot, A., et al.; 2020)
Fig 5. Schematic setup of a Membrane Inlet Mass Spectrometry. (Burlacot, A., et al.; 2020) Fig 6. Overview of designed procedure recommended for extraction and preparation of amino acids from sediments for measurement of radiocarbon isotopic composition. (Blattmann, T. M., et al.; 2020)
Fig 6. Overview of designed procedure recommended for extraction and preparation of amino acids from sediments for measurement of radiocarbon isotopic composition. (Blattmann, T. M., et al.; 2020) Fig 7. Profiling of Amino Acids in Urine Samples of Patients Suffering from Inflammatory Bowel Disease. (Piestansky, J., et al.; 2019)
Fig 7. Profiling of Amino Acids in Urine Samples of Patients Suffering from Inflammatory Bowel Disease. (Piestansky, J., et al.; 2019)