Introduction to Recombinant Human Deoxyribonuclease I
Recombinant Human Deoxyribonuclease I (rhDNase I), also known as Pulmozyme, is a bioengineered version of a naturally occurring human enzyme referred to as human deoxyribonuclease I. Its production involves a procedure known as recombinant DNA technology, where DNA coding for the enzyme is inserted into a host organism, frequently bacteria or yeast, to facilitate the synthesis of the enzyme on a substantial scale.
The primary application of rhDNase I is as a therapeutic measure in managing cystic fibrosis, a hereditary disease characterized by the generation of abnormally viscous and adhesive mucus that can inflict damage on body organs, particularly the lungs. The accumulation of viscous mucus in the lung passages can facilitate bacterial growth by providing an ideal environment, thus leading to infections.
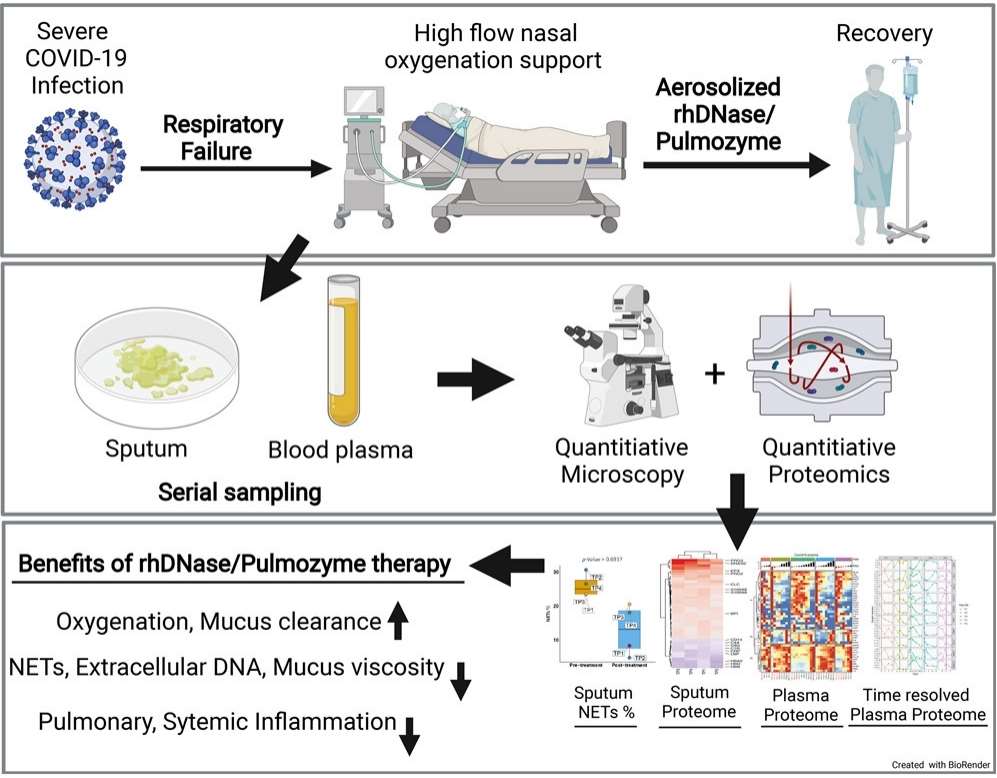 Fig 1. Proteome Profiling of Recombinant DNase Therapy in Reducing NETs and Aiding Recovery in COVID-19 Patients. (Fisher, Jane, et al. 2021)
Fig 1. Proteome Profiling of Recombinant DNase Therapy in Reducing NETs and Aiding Recovery in COVID-19 Patients. (Fisher, Jane, et al. 2021)
Functioning by fragmenting the DNA contained within these mucus secretions, rhDNase I lessens their viscosity, enabling individuals to expectorate them more efficiently. This activity serves to enhance lung function and decrease infection vulnerability. Its delivery via inhalation, often through a nebulizer, ensures the enzyme directly accesses the mucus in the lungs.
Primary Structure of rhDNase and Homology with bDNase
rhDNase is characterized as a unimolecular polypeptide strand consisting of a total of 260 amino acid residues. The primary amino acid sequence for bovine Deoxyribonuclease (bDNase) was previously identified to comprise 257 different amino acid residues. Interestingly, despite the notable divergence in their amino acid sequence homology, the relative propensities for hydrophilicity and hydrophobicity are significantly comparable for each of the two bio-structured entities. Positionally, it has been found that the four cysteine residues maintain the same proximal locations within the sequence alignment for both proteins, which proposes the likely occurrence of identical disulfide bond formations within each. Definitive evidence endorsing this concept was later obtained through a comprehensive application of tryptic digestion and ensuing mass spectrometry, performed under both non-reducing and reducing conditions.
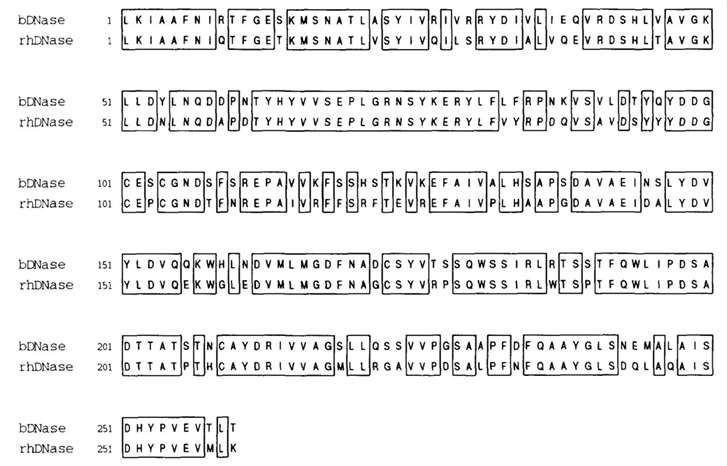 Fig 2. Amino acid sequence homology of bovine (bDNase) and recombinat human (hDNase) deoxyribonuclease l. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 2. Amino acid sequence homology of bovine (bDNase) and recombinat human (hDNase) deoxyribonuclease l. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Secondary and Tertiary Structure
As stated in our prior conversation, analogous structural attributes are likely to be exhibited by both rhDNase and bDNase at the tertiary level. X-ray diffraction, conducted on single crystals, unveiled the three-dimensional layout of bDNase. This protein presents as a compact molecule, with maximal measurements approximating to 45 × 40 × 35 Å. A unique carbohydrate chain, extending approximately 15 Å from the surface at helix I's commencement, also forms part of the molecular structure. This protein subtype falls within the αβ spectrum, typified by two 6-stranded β-pleated sheets, closely bundled into a hydrophobic central core. Extensive loop region formations help flank these aligned β-sheets on both sides.
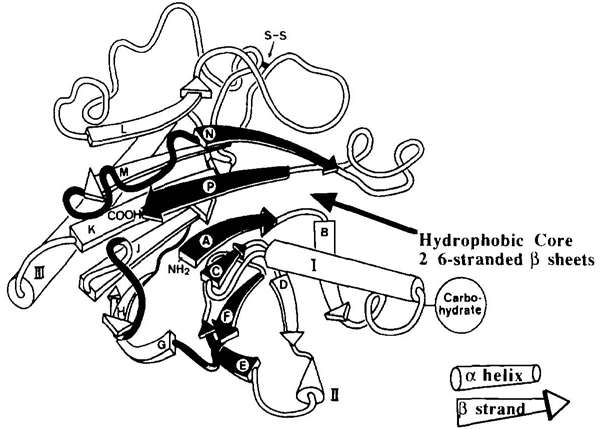 Fig 3. Ribbon diagram depicting the crystal structure of bDNase. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Ribbon diagram depicting the crystal structure of bDNase. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Effect of Bivzlent Matel Ions on Stability
Investigations have demonstrated that the conformal attributes and stability of bDNase have a high degree of dependency on calcium binding. The protein's substantial stability is attributed to its compact, rigid structure. Calcium ions play a pivotal role in preserving this intricate structure. Under the influence of calcium, a reduction can occur in the short disulfide bond between cysteine residues, while the disulfide bond that exists between Cys170 and Cys206 shows notable resistance to reduction.
What Can We Offer?
Utilizing advanced methodologies and state-of-the-art technologies, Creative Proteomics provides a comprehensive array of services for the characterization of protein drugs. Our researchers, all specialists in their respective disciplines, assure meticulous and firm analyses, thereby escalating the intricacy of discerning the features, attributes, and functions of protein-based drugs. Should you wish to delve deeper into the range of our services, kindly feel free to reach out to us.
References
- Fisher, Jane, et al. Proteome profiling of recombinant DNase therapy in reducing NETs and aiding recovery in COVID-19 patients. Molecular & Cellular Proteomics. 2021, 20.
- Pearlman, Rodney, and Y. John Wang, eds. Formulation, characterization, and stability of protein drugs. Vol. 9. Springer Science & Business Media. 1996.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. Proteome Profiling of Recombinant DNase Therapy in Reducing NETs and Aiding Recovery in COVID-19 Patients. (Fisher, Jane, et al. 2021)
Fig 1. Proteome Profiling of Recombinant DNase Therapy in Reducing NETs and Aiding Recovery in COVID-19 Patients. (Fisher, Jane, et al. 2021) Fig 2. Amino acid sequence homology of bovine (bDNase) and recombinat human (hDNase) deoxyribonuclease l. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 2. Amino acid sequence homology of bovine (bDNase) and recombinat human (hDNase) deoxyribonuclease l. (Pearlman, Rodney, and Y. John Wang, eds. 1996) Fig 3. Ribbon diagram depicting the crystal structure of bDNase. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. Ribbon diagram depicting the crystal structure of bDNase. (Pearlman, Rodney, and Y. John Wang, eds. 1996)