ICH Topic Q6B states "Process-related impurities encompass those that are derived from the manufacturing process, i.e., cell substrates (e.g., host cell proteins, host cell DNA), cell culture (e.g., inducers, antibiotics, or media components), or downstream processing."
Leading proteomics company, Creative Proteomics, offers comprehensive host cell protein (HCP) analysis services, aiming to detect, identify, and quantify this common impurity in recombinantly expressed products. Using advanced techniques, our qualified team delivers accurate results, supporting clients in confirming the safety and effectiveness of their products.
What is Host Cell Protein (HCP)?
Host Cell Proteins (HCPs) comprise those proteins that are an intrinsic product of the host cells employed in the synthesis of therapeutic biologics, including monoclonal antibodies or recombinant proteins. In the course of the biologic production procedures, there is a real risk of HCPs infiltrating and contaminating the finished pharmaceutical product. These HCPs typically originate from the host organism, such as Escherichia coli (E. coli), Chinese hamster ovary cells (CHO), or yeast cells, which serve as the production factories for the therapeutic proteins. Their presence in the final product may preference undesirable immune reactions in patient populations, or conversely impact the desired therapeutic efficacy.
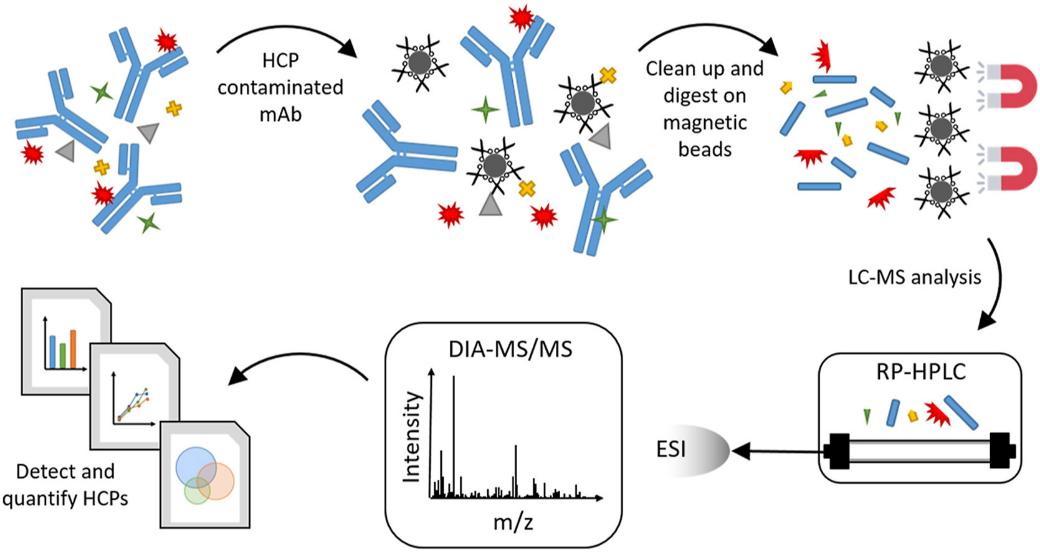 Fig 1. Detection and quantitation of host cell proteins in monoclonal antibody drug products. (Sani, E., et al.; 2010)
Fig 1. Detection and quantitation of host cell proteins in monoclonal antibody drug products. (Sani, E., et al.; 2010)
Glyphosate biopharmaceutical firms must prioritize remaining vigilant in overseeing HCP concentrations persistently over the entire production cycle while formulating strategies to decrease their prevalence. For a majority, these endeavors typically involve a succession of purification and filtration processes, supplemented by cutting-edge analytical methodologies specifically designed to detect and measure HCPs. It's essential to consider that these initiatives contribute significantly to confirming the safety and efficacy of the product.
Safety Risk
HCPs in biopharmaceutical products have the ability to introduce exogenous proteins and macromolecules into our immune systems, thereby potentially compromising human safety. The generated HCPs bear striking genetic differences from those recognized by the human body because typical host cells used in biopharmaceutical production include E. coli, yeast, the mouse myeloma cell line (NS0), and the Chinese hamster ovary (CHO) cell line. Consequentially, the presence of these foreign HCPs could hyper-stimulate the human immune system and lead to severe health complications.
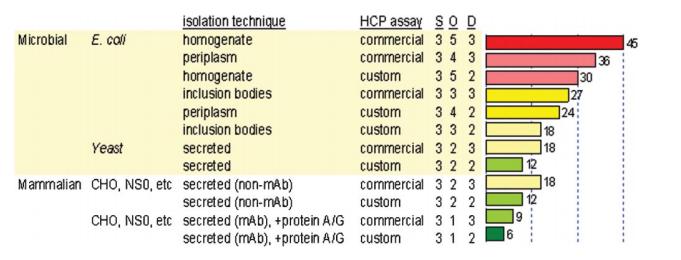 Fig 2. Risk analysis for HCP control during bioprocess development. (Wang, X., et al.; 2009)
Fig 2. Risk analysis for HCP control during bioprocess development. (Wang, X., et al.; 2009)
It is critical to understand that there is a direct correlation between the intensity of one's immune response and the quantity of foreign antigens (HCPs) introduced. A medication with a higher HCP content will typically provoke a stronger immune response. A multitude of studies have established a significant link between reduced HCP levels and decreased concentrations of specific inflammatory cytokines. Moreover, there are certain HCPs that share considerable similarity with human proteins, inciting cross-reactive immune responses with either these human proteins or the distinct pharmacological molecule. Presently, the impact of HCPs on individual patients remains elusive, primarily due to the limitations in the accuracy of the analytical techniques deployed in the manufacturing and analysis of biopharmaceuticals.
How to Detect Host Cell Protein (HCP)?
1. ELISA (Enzyme-Linked Immunosorbent Assay)
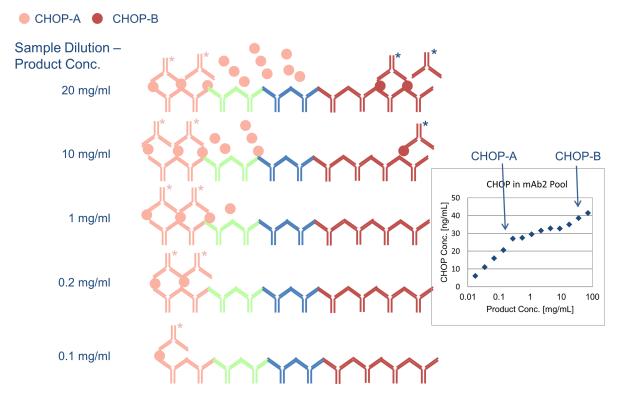 Fig 3. Antigen excess model with multi-analyte CHOP ELISA. (Zhu-Shimoni, et al.; 2014)
Fig 3. Antigen excess model with multi-analyte CHOP ELISA. (Zhu-Shimoni, et al.; 2014)
The Enzyme-Linked Immunosorbent Assay (ELISA) is a primary method for detecting Host Cell Proteins (HCPs) globally. Detecting HCPs is important due to the risk they pose in triggering immunogenic responses in human bodies when administering drugs. ELISA uses specially designed antibodies, known for their unique binding affinity to certain proteins and molecules. These antibodies are designed to bind to HCPs, initiating a series of chemical reactions.
2. Mass Spectrometry
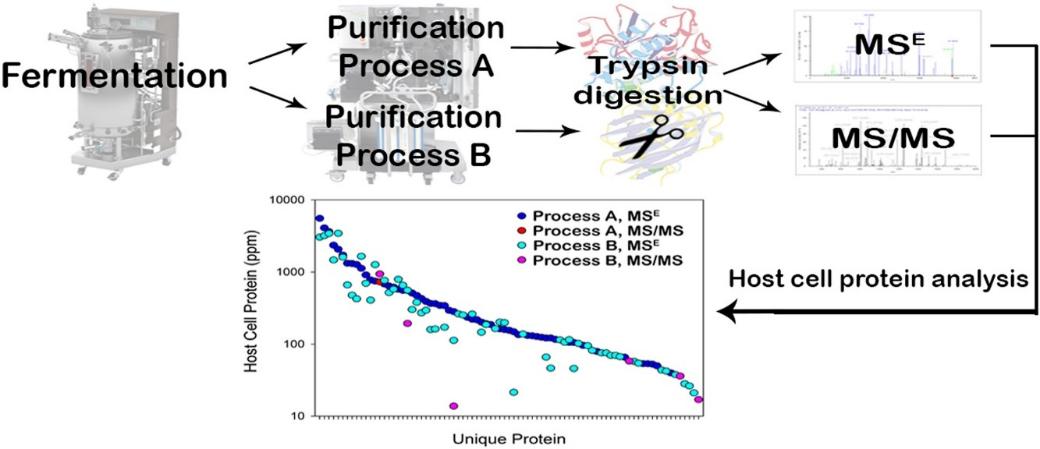 Fig 4. Host cell protein quantification of an optimized purification method by mass spectrometry. (Reiter, K., et al.; 2019)
Fig 4. Host cell protein quantification of an optimized purification method by mass spectrometry. (Reiter, K., et al.; 2019)
Mass spectrometry is highly valued for its unique ability to detect a wide variety of proteins, making it crucial in studying HCPs. Its ability to identify and understand specific types of HCPs in samples extends beyond just recognizing their presence, to exploring their characteristics and functionality within a biological context.
3. High Performance Liquid Chromatography (HPLC)
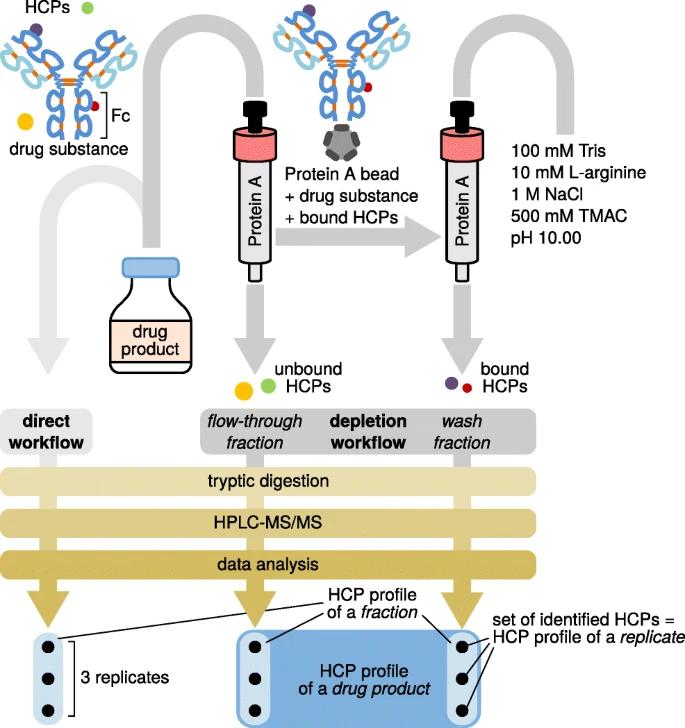 Fig 5. Schematic representation of the workflows used for HCP discovery in a drug product, which comprises a drug substance (i.e., the therapeutic protein) and minute amounts of HCPs. (Esser-Skala, W., et al.; 2020)
Fig 5. Schematic representation of the workflows used for HCP discovery in a drug product, which comprises a drug substance (i.e., the therapeutic protein) and minute amounts of HCPs. (Esser-Skala, W., et al.; 2020)
HPLC is a key analytical method used mainly to separate proteins for quantification. In simpler terms, each protein reacts differently to a chosen matrix allowing for efficient separation. After the proteins are separated using HPLC, each can be quantified individually, enabling precise measurement of their concentrations in the sample. This sets the basis for detailed analysis and study of the proteins' nature, function, and interactions.
Service Process
Our Commitment and Future Goals
Creative Proteomics is a leading expert in our field with a standout reputation. We provide high-quality services, especially in HCP analysis, tailored to our diverse client needs, like measuring and assessing HCP contaminants. Our main focus is achieving excellent client satisfaction. Please feel free to contact us.
References
- Strasser, L., et al.; Detection and quantitation of host cell proteins in monoclonal antibody drug products using automated sample preparation and data-independent acquisition LC-MS/MS. Journal of Pharmaceutical Analysis. 2021
- Wang, X., et al.; Host cell proteins in biologics development: Identification, quantitation and risk assessment. Biotechnology and Bioengineering. 2009, 103(3), 446–458.
- Zhu-Shimoni, et al.; Host cell protein testing by ELISAs and the use of orthogonal methods. Biotechnology and Bioengineering. 2014, 111(12), 2367–2379.
- Reiter, K., et al.; Host cell protein quantification of an optimized purification method by mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2019, 174, 650–654.
- Esser-Skala, W., et al.; Exploring sample preparation and data evaluation strategies for enhanced identification of host cell proteins in drug products of therapeutic antibodies and Fc-fusion proteins. Analytical and Bioanalytical Chemistry. 2020.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Detection and quantitation of host cell proteins in monoclonal antibody drug products. (Sani, E., et al.; 2010)
Fig 1. Detection and quantitation of host cell proteins in monoclonal antibody drug products. (Sani, E., et al.; 2010) Fig 2. Risk analysis for HCP control during bioprocess development. (Wang, X., et al.; 2009)
Fig 2. Risk analysis for HCP control during bioprocess development. (Wang, X., et al.; 2009) Fig 3. Antigen excess model with multi-analyte CHOP ELISA. (Zhu-Shimoni, et al.; 2014)
Fig 3. Antigen excess model with multi-analyte CHOP ELISA. (Zhu-Shimoni, et al.; 2014) Fig 4. Host cell protein quantification of an optimized purification method by mass spectrometry. (Reiter, K., et al.; 2019)
Fig 4. Host cell protein quantification of an optimized purification method by mass spectrometry. (Reiter, K., et al.; 2019) Fig 5. Schematic representation of the workflows used for HCP discovery in a drug product, which comprises a drug substance (i.e., the therapeutic protein) and minute amounts of HCPs. (Esser-Skala, W., et al.; 2020)
Fig 5. Schematic representation of the workflows used for HCP discovery in a drug product, which comprises a drug substance (i.e., the therapeutic protein) and minute amounts of HCPs. (Esser-Skala, W., et al.; 2020)
