Introduction to Recombinant Human G-CSF
Neutropenia, a medical condition distinguished by a decline in neutrophil concentrations under the standard range, approximated at 1.5 ×109/L, manifests itself across a multitude of disease circumstances, incorporating both hereditary disorders and infection. The condition is particularly prevalent in cancer patients subjected to cytotoxic chemotherapy. A notable consequence of neutropenia is a heightened vulnerability to bacterial and subsequent fungal infections, frequently necessitating hospital admission.
One primary therapeutic application of granulocyte-colony stimulating factor (G-CSF) centers on counteracting neutropenia. The administration of G-CSF possesses the capacity to either curtail the duration of neutropenia or preclude it completely. Moreover, treatment by means of G-CSF can facilitate the execution of higher-intensity dosing schedules, possibly enhancing the efficacy of antitumor undertakings.
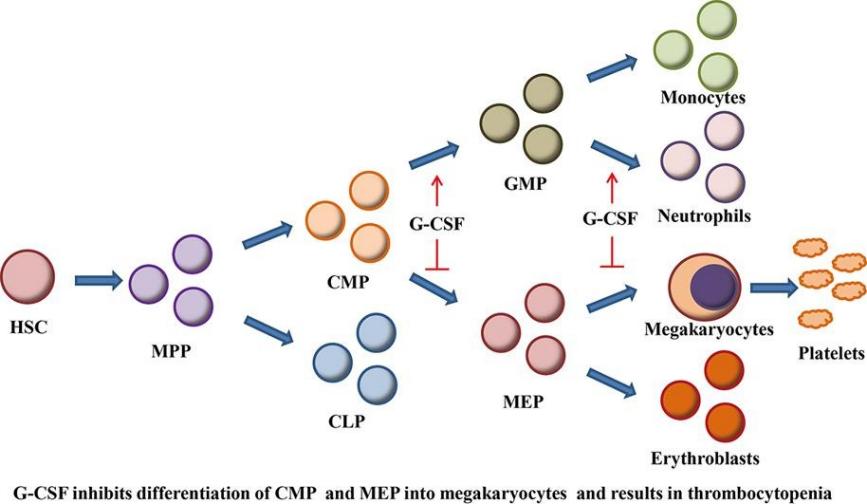 Fig 1. G-CSF administration results in thrombocytopenia by inhibiting the differentiation of hematopoietic progenitors into megakaryocytes. (Li, Yunqian, et al. 2019)
Fig 1. G-CSF administration results in thrombocytopenia by inhibiting the differentiation of hematopoietic progenitors into megakaryocytes. (Li, Yunqian, et al. 2019)
In recognition of its potential to alleviate neutropenia, especially during or subsequent to chemotherapy, the pharmaceutical agent Neupogen® was authorized for use by the United States Food and Drug Administration in 1991. The medication has, in the intervening years, achieved approval from a multitude of global regulatory bodies.
Primary Structure of G-CSF
Neupogen® is comprised of a 175-amino acid polypeptide chain. When in solution, this FDA-approved drug presents as a monomeric molecule and has a molecular weight of 18,799.94, as deduced from its genomic sequence. Employing N-terminal sequence analysis on purified G-CSF, methionine functions as the singular amino terminus. This insinuates that unlike other proteins, the initiator of protein synthesis in this context does not necessarily experience post-translational processing.
 Fig 2. Overlay of wild type G-CSF (PDB Code: 1CD9) with the representative structure of most populated cluster resulted from 100 ns simulation of M1 and M2 mutant. (Mishra, Avinash, et al. 2020)
Fig 2. Overlay of wild type G-CSF (PDB Code: 1CD9) with the representative structure of most populated cluster resulted from 100 ns simulation of M1 and M2 mutant. (Mishra, Avinash, et al. 2020)
The bioactive conformation of G-CSF is preserved by the formation of two compact loop structures resultant of two intramolecular disulfide bonds, specifically, Cys37-Cys43 and Cys65-Cys75. A reductive alteration in any of these disulfide bonds can significantly hinder the protein's overall activity. Moreover, Neupogen® incorporates a solo cysteine at the 18th position which, interestingly, is not a prerequisite for its bioactivity.
Complete Amino Acid Sequence
Neupogen® was subjected to in-depth structural characterization to accurately determine its chemical composition. Amino acid analysis and peptide mapping were performed using a variety of analytical techniques and the amino acid sequence was determined. S-carboxymethylated Neupogen® was digested with the endoprotease Glu-C to generate peptide fragments. In addition, the complete protein was digested using chymotrypsin. After digestion, these fragments were separated by reversed-phase high-performance liquid chromatography (HPLC). After cleavage with the endoprotease Glu-C, a typical peptide map of alkylated Neupogen® was drawn, and this method has been the reference for our product characterization tests.
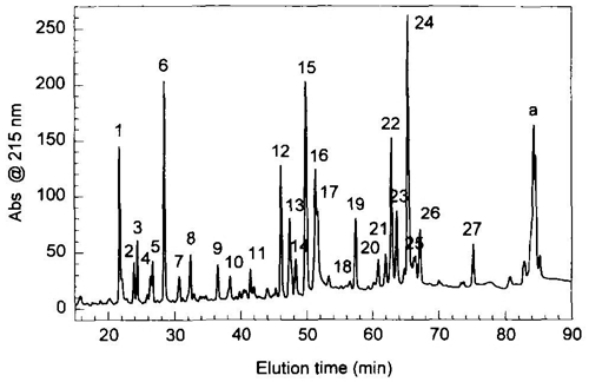 Fig 3. HPLC peptide map of reduced and carboxymethylated Neupogen® after endoproteinase Glu-C digestion. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. HPLC peptide map of reduced and carboxymethylated Neupogen® after endoproteinase Glu-C digestion. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
What Can We Offer?
Through the adept utilization of advanced methodologies and revolutionary technologies, Creative Proteomics proffers an extensive repertoire of all-encompassing protein drug characterization services. Our accomplished team of scientists with profound expertise ensures the provision of precise and dependable analyses, facilitating an intricate understanding of the attributes, qualities, and mechanisms of protein drugs. Please do not hesitate to get in touch with us to explore our services further.
References
- Li, Yunqian, et al. G-CSF administration results in thrombocytopenia by inhibiting the differentiation of hematopoietic progenitors into megakaryocytes. Biochemical Pharmacology. 2019, 169: 113624.
- Mishra, Avinash, et al. Mechanistic explanation of structural and functional changes induced by methionine mutation in G-CSF protein. Current Research in Biotechnology. 2020, 2: 37-44.
- Pearlman, Rodney, and Y. John Wang, eds. Formulation, characterization, and stability of protein drugs. Vol. 9. Springer Science & Business Media. 1996.
Related Sections
Services
Applications
Creative Proteomics specializes in protein drug characterization, and we offer a range of services to help our clients understand and optimize their protein drug products. Our services include, but are not limited to:
|
Protein Drug characterization
|
Reaearch Project
|
Method
|
Application
|
|
Protein Structure Confirmation Service
|
Primary Structure Analysis
|
X-ray crystal diffraction,
nuclear magnetic resonance (NMR) spectroscopy,
ellman's assay,
ion exchange chromatography (IEC),
edman degradation,
mass spectrometry (MS), etc.
|
Protein functions, disease mechanisms, and drug design, etc.
|
|
Higher-Order Structure Analysis
|
|
Post-Translational Modification (PTM) Analysis Service
|
Protein Glycan Analysis
|
Mass spectrometry (MS),
nuclear magnetic resonance (NMR) spectroscopy,
lectin affinity chromatography,
liquid chromatography-mass spectrometry (LC-MS), etc.
|
Disease mechanism research, drug discovery and development, regulation of biological processes, clinical diagnostic, bioinformatics, etc.
|
|
Protein Acetylation Analysis
|
|
Protein Phosphorylation Analysis
|
|
Protein Ubiquitination Analysis
|
|
Protein Deamidation Analysis
|
|
Protein Oxidation Analysis
|
|
Protein Methylation Analysis
|
|
Protein Alkylation Analysis
|
|
Protein Sulfation Analysis
|
|
Proteolysis Analysis
|
|
Protein Truncation Analysis
|
|
Protein Physicochemical Property Determination Service
|
Isoelectric Point (PI) Determination
|
Isoelectric focusing (IEF),
thioflavin T (ThT) fluorescence assay,
western blotting,
ultraviolet-visible spectrometry,
fluorescence spectrometry, etc.
|
Protein structure study, protein interaction study, protein modification study, protein purity and quantitative analysis, clinical diagnosis, etc.
|
|
Charge Variant Analysis
|
|
Extinction Coefficient Determination
|
|
Protein Aggregation Analysis
|
|
Protein Degradation Analysis
|
|
Thermal (Tm) Stability Analysis
|
|
Protein Quantitation
|
|
Protein Purity Service
|
/
|
Composition-based and activity-based analyses,
mass spectrometry (MS),
high performance liquid chromatography,
capillary electrophoresis, etc.
|
Biomedical research, biological research, clinical diagnostics, etc.
|
|
Protein Impurities Service
|
Host Cell Protein (HCP) Analysis
|
High performance liquid chromatography (HPLC),
mass spectrometry (MS),
size-exclusion chromatography (SEC),
capillary electrophoresis, etc.
|
Quality control, safety assessment, clinical diagnostics, bioprocess research, etc.
|
|
Residual Host Cell DNA (HCD) Analysis
|
|
Residual Protein A Analysis
|
|
Process Related Impurities and Residual Analysis
|
|
Protein Biosafety Analysis Service
|
Bacterial Endotoxins Testing
|
Limulus amebocyte lysate (LAL),
membrane filtration,
PCR,
nucleic acid testing (NAT),
massively parallel sequencing (MPS), etc.
|
Biomedical research, food safety testing, environmental science, industrial production, etc.
|
| Bioburden Testing |
| Sterility Testing |
| Abnormal Toxicity Testing |
| Mycoplasma Testing |
| Subvisible Particles Analysis |
| Visible Particles Analysis |
For research use only, not intended for any clinical use.


 Fig 1. G-CSF administration results in thrombocytopenia by inhibiting the differentiation of hematopoietic progenitors into megakaryocytes. (Li, Yunqian, et al. 2019)
Fig 1. G-CSF administration results in thrombocytopenia by inhibiting the differentiation of hematopoietic progenitors into megakaryocytes. (Li, Yunqian, et al. 2019) Fig 2. Overlay of wild type G-CSF (PDB Code: 1CD9) with the representative structure of most populated cluster resulted from 100 ns simulation of M1 and M2 mutant. (Mishra, Avinash, et al. 2020)
Fig 2. Overlay of wild type G-CSF (PDB Code: 1CD9) with the representative structure of most populated cluster resulted from 100 ns simulation of M1 and M2 mutant. (Mishra, Avinash, et al. 2020) Fig 3. HPLC peptide map of reduced and carboxymethylated Neupogen® after endoproteinase Glu-C digestion. (Pearlman, Rodney, and Y. John Wang, eds. 1996)
Fig 3. HPLC peptide map of reduced and carboxymethylated Neupogen® after endoproteinase Glu-C digestion. (Pearlman, Rodney, and Y. John Wang, eds. 1996)