The ICH Q6B guideline clearly requires that modifications to be identified in biological products. In order to assist clients across the world in researching protein structure and function as well as the mechanisms underlying the beginning and progression of associated disorders, Creative Proteomics offers protein deamidation analysis services.
What is Protein Deamidation?
Protein deamidation is a chemical modification process where the amide group (-CONH2) in the side chain of asparagine (Asn) or glutamine (Gln) residues is converted to a carboxylic acid group (-COOH). This conversion occurs through the hydrolysis of the amide bond, resulting in the loss of ammonia (NH3) and the addition of a carbonyl group (C=O).
Deamidation of proteins can occur spontaneously under physiological conditions, but it can also be accelerated by factors such as temperature, pH, and the presence of certain chemicals. The rate of deamidation varies depending on the specific protein and amino acid sequence, with Asn deamidating faster than Gln.
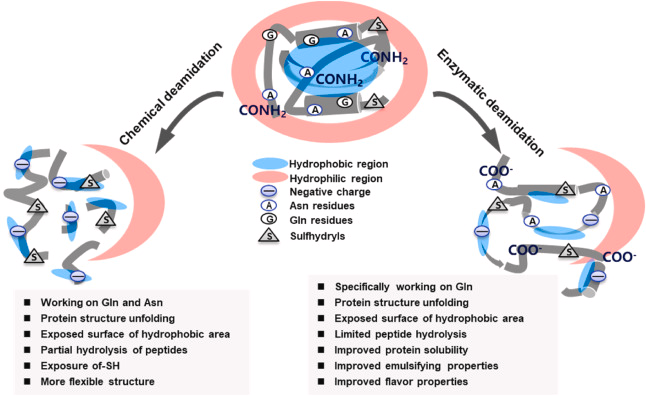 Fig 1. Deamination mechanism by chemical and PG methods and its effect on protein properties. (Zhang, G., et al.; 2021)
Fig 1. Deamination mechanism by chemical and PG methods and its effect on protein properties. (Zhang, G., et al.; 2021)
Protein deamidation can have functional consequences on protein structure and function. It can lead to changes in charge and polarity of the affected residues, potentially affecting protein-protein interactions, enzymatic activities, conformational stability, and immune recognition. In some cases, deamidation may contribute to protein degradation and loss of protein functionality. Therefore, protein deamidation is an important consideration in biopharmaceuticals, food processing, and aging-related protein dysfunction studies.
Mechanism for Spontaneous Deamidation
Proteins' internal asparagine residues undergo non-enzymatic deamidation in two phases by an intramolecular rearrangement that takes place close to neutral pH. The first amino acid residue exactly adjacent to the C-terminal end of the asparagine or glutamine, also known as the N + 1 amino acid, attacks the carbonyl carbon of the side chain to generate a cyclic imide in the first, rate-limiting step.
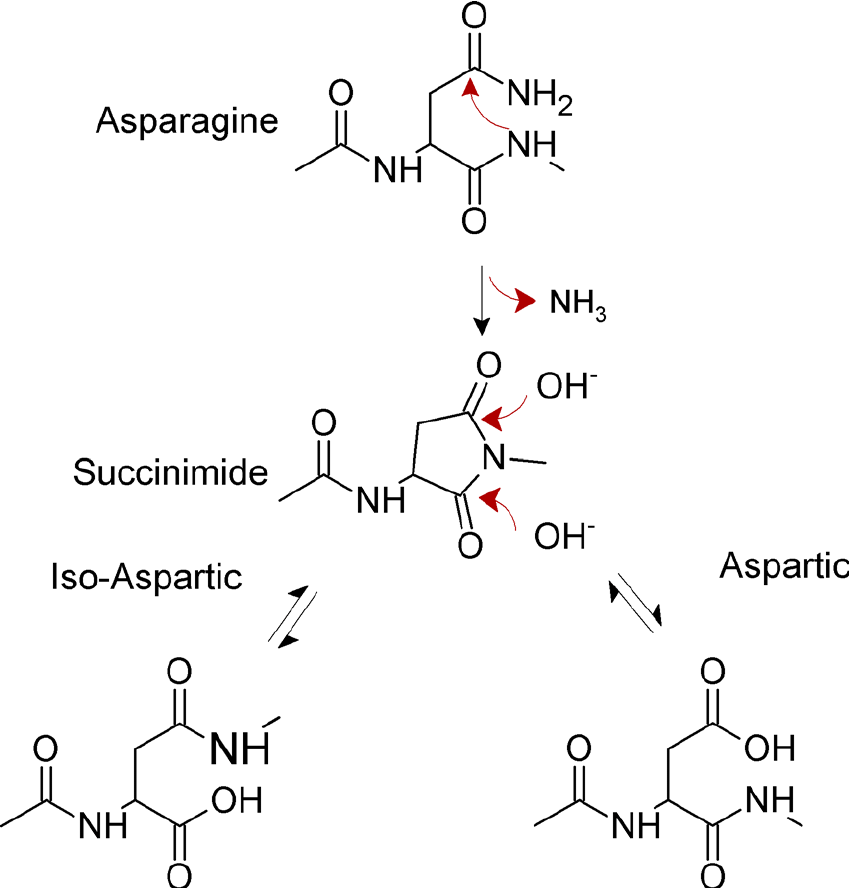 Fig 2. Mechanism for spontaneous deamidation of internal asparagine residues in proteins. (Lorenzo, J. R., et al.; 2015)
Fig 2. Mechanism for spontaneous deamidation of internal asparagine residues in proteins. (Lorenzo, J. R., et al.; 2015)
Application of Protein Deamidation
Biopharmaceuticals
Protein deamidation is a critical attribute to monitor in the development and manufacturing of biopharmaceuticals, such as monoclonal antibodies and recombinant proteins. It can impact the safety, efficacy, and stability of these therapeutic proteins.
Food Science
Deamidation of food proteins affects their functional properties, such as solubility, gelation, and emulsification ability. Understanding and controlling deamidation is important for improving the quality and shelf life of food products.
Aging & Disease
Protein deamidation is known to accumulate over time, especially in long-lived proteins. Studying deamidation can provide insights into the aging process and disease mechanisms.
Protein Research
Deamidation can alter the conformation and interactions of proteins, affecting their biochemical properties and biological functions. Investigating protein deamidation helps elucidate structure-function relationships and protein dynamics.
How to Detect Protein Deamidation?
Mass Spectrometry
- Mass spectrometry (MS) is widely used for the identification and quantification of post-translational modifications including deamidation. By measuring the mass-to-charge ratio of peptides, mass spectrometry can accurately detect and quantify deamidated species.
Antibody-Based Assays
- Using antibodies that specifically recognize deamidated peptides, researchers can easily detect the presence of deamidated proteins in a sample. Techniques such as enzyme-linked immunosorbent assays (ELISA) and Western blotting are commonly used for this purpose.
Capillary Electrophoresis
- The charge difference caused by deamidation alters the migration of proteins in the capillary and is suitable for distinguishing between deamidated and non-deamidated proteins. By comparing the mobility of the two forms, the degree of deamidation can be determined.
Liquid Chromatography
- Reversed-phase or ion-exchange chromatography coupled with mass spectrometry allows researchers to overcome the challenges posed by complex protein mixtures and accurately identify and quantify deamidated species.
Nuclear magnetic resonance (NMR) spectroscopy
- Changes in chemical shifts or relaxation rates indicate the presence of deamidated residues, providing researchers with a way to analyze the effects of deamidation on protein structure and function.
Our Advantages

Our Goals
Creative Proteomics is your reliable partner if you need cutting-edge expertise in protein drug characterisation. Our industry-leading services and considerable experience in this field can considerably accelerate your medication development process and ensure your protein drug's success. Please contact us to learn more about our services and how we can help you maximize the potential of your protein drugs.
References
- Zhang, G., et al.; Protein-glutaminase: Research progress and prospect in food manufacturing. Food Bioscience. 2021, 43, 101314.
- Lorenzo, J. R., et al.; Prediction of Spontaneous Protein Deamidation from Sequence-Derived Secondary Structure and Intrinsic Disorder. PLOS ONE. 2015, 10(12), e0145186.
Related Sections
Services
Applications
For research use only, not intended for any clinical use.


 Fig 1. Deamination mechanism by chemical and PG methods and its effect on protein properties. (Zhang, G., et al.; 2021)
Fig 1. Deamination mechanism by chemical and PG methods and its effect on protein properties. (Zhang, G., et al.; 2021) Fig 2. Mechanism for spontaneous deamidation of internal asparagine residues in proteins. (Lorenzo, J. R., et al.; 2015)
Fig 2. Mechanism for spontaneous deamidation of internal asparagine residues in proteins. (Lorenzo, J. R., et al.; 2015)