- Service Details
- Case Study
What is O-Glycan Liberation?
O-Glycan liberation refers to the process of releasing O-glycans from glycoproteins. O-glycans are complex carbohydrate structures that are covalently attached to proteins through O-glycosidic linkages. These linkages typically involve the hydroxyl groups of serine or threonine residues in the protein backbone.
The liberation of O-glycans is an essential step in glycomics research, which involves the study of the structure and function of carbohydrates (glycans) in biological systems. Researchers often perform O-glycan liberation to isolate and analyze the composition of O-glycans from glycoproteins. This process allows for the characterization of the specific carbohydrate structures present, aiding in understanding their roles in various biological processes.
Methods for O-glycan liberation may involve enzymatic or chemical treatments to cleave the O-glycans from the protein, making them available for further analysis by techniques such as mass spectrometry or chromatography. Studying O-glycans is important in elucidating their significance in cell signaling, immune response, and various physiological functions.
Methods of O-Glycan Liberation
Various methods, each possessing unique advantages and considerations, have been developed to facilitate the strategic extraction of O-glycans from glycoproteins. Creative Proteomics, as a leading provider of O-glycan liberation services, excels in utilizing diverse methodologies to meet specific experimental requirements.
Enzymatic Cleavage: PNGase F
PNGase F is an enzyme known for its specificity in cleaving the N-glycosidic bond between glycans and asparagine residues. While traditionally associated with N-glycan release, PNGase F can be strategically employed for selective O-Glycan Liberation.
Process:
- Incubation of glycoproteins with PNGase F.
- Specific cleavage of the glycosidic bond.
- Liberation of O-glycans from the glycoprotein.
Advantages:
- High specificity for N-glycosidic bonds.
- Minimal impact on O-glycan structures.
- Compatibility with various glycoproteins.
Chemical Approaches: Beta-Elimination
Beta-elimination involves the chemical cleavage of O-glycans through the elimination of a hydroxyl group. This method is versatile and applicable to a broad range of O-glycan structures.
Process:
- Treatment of glycoproteins with a beta-elimination reagent.
- Chemical cleavage of O-glycans.
- Release of O-glycans for subsequent analysis.
Advantages:
- Applicability to diverse glycoprotein structures.
- Versatility in cleaving various types of O-glycans.
Chemical Approaches: Hydrazinolysis
Hydrazinolysis involves the chemical cleavage of O-glycans through the use of hydrazine, ensuring effective liberation.
Process:
- Incubation of glycoproteins with hydrazine.
- Chemical cleavage of O-glycans.
- Liberation of O-glycans for downstream analysis.
Advantages:
- Effective cleavage of O-glycans with minimal side reactions.
- Compatibility with diverse glycoprotein structures.
Our experts may employ a strategic combination of enzymatic and chemical methods for enhanced O-Glycan Liberation, ensuring a comprehensive extraction suited to the unique characteristics of your samples.
Why Choose Us?
Cutting-Edge Technologies: Employing cutting-edge technologies, we've achieved a remarkable 98% success rate in O-glycan liberation. Our state-of-the-art methodologies ensure precision and efficiency, guaranteeing optimal results for your glycomic analyses.
Proven Track Record: Trust in our proven track record backed by over 150 successful glycomic projects. From elucidating disease-specific glycosylation patterns to aiding in biomarker discovery, our portfolio speaks volumes about our ability to deliver impactful results.
Tailored Approaches: Recognizing the uniqueness of each glycoprotein, we offer 100% personalized approaches. Our scientists carefully tailor liberation methods based on glycoprotein characteristics, ensuring that your project receives the attention it deserves.
Comprehensive Support: Experience comprehensive support with our round-the-clock assistance. Whether it's clarifying sample requirements, addressing queries, or ensuring seamless communication, our dedicated support team is available 24/7 to cater to your needs.
Applications of O-Glycan Liberation
Structural Elucidation: Examine the detailed structural composition of O-glycans in isolation, providing insights into their molecular architecture.
Functional Characterization: Unravel the intricate roles of liberated O-glycans in biological processes, particularly in cellular recognition and signaling pathways.
Disease-Specific Glycosylation Analysis: Investigate and decode disease-specific glycosylation patterns, contributing to the discovery of potential biomarkers for disease diagnosis and prognosis.
Identification of Therapeutic Targets: Identify specific O-glycan structures associated with diseases, facilitating the pinpointing of potential therapeutic targets for precision medicine interventions.
Glycoprotein Engineering Optimization: Leverage liberated O-glycans for optimizing glycoprotein engineering processes, enhancing functionality for applications in pharmaceuticals and biotechnology.
Cell Signaling Dynamics: Contribute to a nuanced understanding of cell signaling intricacies through comprehensive analysis of O-glycans involved in signaling cascades.
Advancements in Functional Glycomics: Drive progress in functional glycomics by dissecting the nuanced functional significance of glycans across diverse biological contexts.
Comparative Glycomic Analysis: Conduct in-depth comparative glycomic analysis, elucidating variations in O-glycan structures across different samples to discern physiological or pathological states.
Sample Requirements for O-Glycan Liberation
| Sample Type | Reference Quantity |
|---|---|
| Cell Cultures | 1 x 107 cells |
| Tissues | 50 mg |
| Biofluids | 500 µL |
For specific inquiries about additional sample types or any other detailed requirements, please contact us. Our team is ready to provide tailored guidance based on your project's unique aspects.
Case. Glycan Structural Analysis Reveals Differential Expression in PDAC PDX Models Using H-type3-Specific Probe rBC2LCN
Background:
Pancreatic Ductal Adenocarcinoma (PDAC) is a highly aggressive malignancy with a dismal prognosis. Current diagnostic and therapeutic limitations necessitate innovative approaches. Glycosylation pattern alterations in cell surface proteins and secreted glycoproteins present potential targets for drug discovery. Previous studies identified glycan alterations in PDAC, including sialyl Lewis A and other glycans. This study builds upon glycome analysis of PDAC cell lines, emphasizing the significance of glycosylation in PDAC biology.
Samples
Two PDAC Patient-Derived Xenograft (PDX) models, PC3 and PC42, along with Capan-1 cell line samples, were utilized. Tumor tissues from these models served as the primary focus for quantitative structural glycan analysis.
Technical Method
- PDAC PDX Model Generation: Human PDAC tissues implanted into SCID mice, and subsequent generations were established by subcutaneous implantation.
- Cell Culture: Capan-1 cells cultured and recovered for analysis.
- Tissue Staining: Formalin-fixed tissue sections stained with rBC2LCN to visualize glycan ligands.
- Glycan Liberation and Pyridylamination: Glycans released by gas-phase hydrazinolysis, re-N-acetylated, and labeled with 2-aminopyridine.
- HPLC Analysis: Anion-exchange, size-fractionation, and reversed-phase HPLC used for separation and quantification of glycans.
- MALDI-TOFMS: Purified PA-glycans subjected to MALDI-TOFMS analysis for glycan identification.
- Exoglycosidase Treatment: Various exoglycosidases used for enzymatic treatment of PA-glycans.
- Analysis of Sialic Acid Linkage Type: Determination of sialic acid linkage type using specific sialidases and anion-exchange HPLC.
Results
- PDAC PDX models PC3 and PC42 exhibited distinct tumor structures.
- Glycan structural differences observed between PC3 and PC42, including increased sialylated and highly branched N-glycans in PC42.
- Core 3 O-glycan expression was higher in well-differentiated PC3 compared to poorly-differentiated PC42.
- H-type3 glycan epitope of rBC2LCN was detected in both PDX models and Capan-1, suggesting its uniqueness in PDAC.
- Potential therapeutic and diagnostic significance of H-type3 glycan as a novel target for PDAC highlighted, with ongoing technology development.
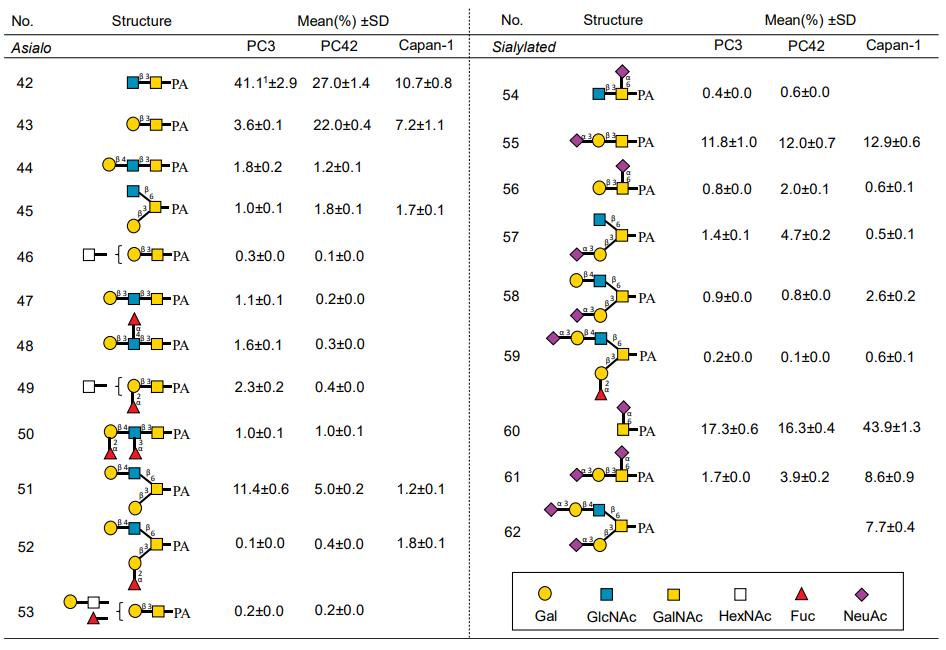 List of O-glycans identified in PC3, PC42, and Capan-1.
List of O-glycans identified in PC3, PC42, and Capan-1.
Reference
- Hasehira, Kayo, et al. "Quantitative structural analysis of glycans expressed within tumors derived from pancreatic cancer patient-derived xenograft mouse models." Biochemical and Biophysical Research Communications 534 (2021): 310-316.