Native Mass Spectrometry
In the past two decades, native mass spectrometry (MS) has been developed as a powerful technique in the field of structural biology of protein assemblies. In general, native MS refers to the process whereby biomolecules and complexes can be transferred from a three-dimensional, functional existence in a condensed liquid phase to the gas phase. Since the experimental conditions are mild, noncovalent interactions, quarternary structural arrangements, and the associated biological action and functionality of protein complexes can be largely preserved. Notably, native MS can be applied to investigate viral host cell protein complexes, including subunit identification, subunit stoichiometry, biomolecular binding, protein dynamics, and so on.
Two key factors to the success of native MS
Native MS is based on electrospray ionization (ESI), which introduces biological analytes into the mass spectrometer using a non-denaturing solvent that preserves the non-covalent interactions holding the three-dimensional structure of the proteins together. In order to ensure the reliable performance of a native MS experiment, two key criteria need to be addressed. The proper sample preparation is often sample-dependent and remains one of the biggest hurdles for many laboratories. The nature of the solution containing the protein complex is crucial. In order to maintain protein complexes' structural integrity and prevent degradation, complexes should be stored under physiologically compatible conditions. And the conditions (usually a volatile ammonium-based semi-buffer) not only maintain the integrity of the interactions but also compatible with the desolvation process required during the transition from liquid to the gas phase. In addition, another key criterion is that instruments with advanced physical features and capabilities should be further developed. Due to the natural folding state of these protein complexes, the number of available sites for acquiring a charge as well as the number of visible charge states in the MS is reduced. Besides, these large supramolecular complexes are characterized by high mass-to-charge (m/z) ratios and therefore are generally not within the mass range of standard mass spectrometers.
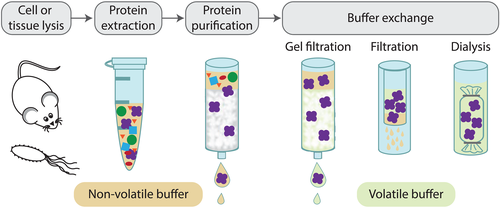 Sample preparation for native MS. (Barth, M., et al, 2020)
Sample preparation for native MS. (Barth, M., et al, 2020)
Native MS-based studies of viruses
Typically, the capsids of icosahedral viruses and virus-like particles (VLPs) assemble from one or different capsid proteins at a well-defined stoichiometry, which makes them tractable targets for native MS. In fact, viruses or virus-like particles (VLPs) are often utilized as a benchmark for the development of new native MS technologies, therefore in this respect, the studies of virus assembly and native MS method development have gone hand in hand in the last several years. So far, with the further improvements and developments in the native MS method, the capsid stability, exchange dynamics, and ligand binding of viruses are accessible. For instance, the establishment of high-resolution native mass spectrometers modified for transmission of high-mass complexes has been a major breakthrough that can provide insight into capsid assembly, capsid composition as well as cargo loading of the viruses.
 The understanding of virus capsid assembly, the exact numbers of protein monomers into the distinct architecture of virus capsids, may promote the development of anti-viral therapeutics. Over the last two decades, instrumentation, sample preparation, and data analysis of native MS have been improved. Creative Proteomics is a leading provider in the field of viral proteomics services. Based on the improved native MS method, we can not only help customers study the composition of viruses, the structural integrity of virions and the stoichiometry of viral proteins, but also detect assembly intermediates and monitor viral assembly.
The understanding of virus capsid assembly, the exact numbers of protein monomers into the distinct architecture of virus capsids, may promote the development of anti-viral therapeutics. Over the last two decades, instrumentation, sample preparation, and data analysis of native MS have been improved. Creative Proteomics is a leading provider in the field of viral proteomics services. Based on the improved native MS method, we can not only help customers study the composition of viruses, the structural integrity of virions and the stoichiometry of viral proteins, but also detect assembly intermediates and monitor viral assembly.
For more information on how we can help you, please feel free to contact us. We look forward to providing services for your next project.
Reference
- Barth, M., & Schmidt, C. (2020). "Native mass spectrometry—A valuable tool in structural biology." Journal of Mass Spectrometry, 55(10), e4578.
* For research use only.

 Sample preparation for native MS. (Barth, M., et al, 2020)
Sample preparation for native MS. (Barth, M., et al, 2020)  The understanding of virus capsid assembly, the exact numbers of protein monomers into the distinct architecture of virus capsids, may promote the development of anti-viral therapeutics. Over the last two decades, instrumentation, sample preparation, and data analysis of native MS have been improved. Creative Proteomics is a leading provider in the field of viral proteomics services. Based on the improved native MS method, we can not only help customers study the composition of viruses, the structural integrity of virions and the stoichiometry of viral proteins, but also detect assembly intermediates and monitor viral assembly.
The understanding of virus capsid assembly, the exact numbers of protein monomers into the distinct architecture of virus capsids, may promote the development of anti-viral therapeutics. Over the last two decades, instrumentation, sample preparation, and data analysis of native MS have been improved. Creative Proteomics is a leading provider in the field of viral proteomics services. Based on the improved native MS method, we can not only help customers study the composition of viruses, the structural integrity of virions and the stoichiometry of viral proteins, but also detect assembly intermediates and monitor viral assembly.