Viral TMT-Based Proteomic Service
Proteomic analysis has become a powerful tool for studying the host cells responding to virus infection, including characterizing cellular proteins that are directly or indirectly involved in virus replication and analyzing virus-host protein interactions to advance the understanding of how viral infection leads to host pathogenicity. Over the past few years, tandem mass tag (TMT) combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis has played an increasingly important role in the identification and quantitation analysis of the proteomic profiles. The method can provide a uniquely accurate quantitative measurement of protein abundances on a near-global proteomic scale and has been successfully used to investigate a variety of viruses, such as human immunodeficiency virus (HIV), canine parvovirus, Bombyx mori nucleopolyhedrovirus, and Epstein-Barr virus.
Creative Proteomics is a forward-looking research institute as well as a leading custom service provider in the field of viral proteomics. We provide custom experimental design and professional technical support according to customers' specific project needs. Based on TMT-based proteomic analysis, we can help global customers to explore the dynamic interactions between virus and host, and understand the pathogenesis involved in viral infection and replication.
How does TMT quantitative proteomics work?
TMT technology was developed by Thermo Fisher Scientific for the identification and quantification of proteins in different kinds of samples. As isobaric chemical tags, the TMT system can provide multiplexing capabilities for relative quantitative proteomics analysis. TMT tagging reagent and iTRAQ reagents are basically similar in structure and detection principle, both of which are composed of three parts, a reporter group, a balanced group, and an amine reactive group. During labeling, the amine reactive group binds to a lysine residue or to the N-terminus of a peptide.
The isobaric label TMT is available in up to 11 tags that can be used for labeling practically any peptide or protein sample. In the experiment of TMT quantitative proteomic, different isobaric tagging reagents are used to label different samples. Then the mixed samples are analyzed in a single liquid chromatography-mass spectrometry (LC-MS). During LC separation, all peptides from different TMT-labeled samples are eluted together because the isobaric tags have the same chemical properties. Therefore, they appear as a single and indistinguishable precursor ion peak in the mass spectrum. Following fragmentation, fragment reporter ions released from the labels are in the low mass region of the MS/MS spectrum. Peptide identification is accomplished by matching fragment databases, while peptide quantitation is performed by measuring the intensities of these reporter ions.
Examples of TMT-coupled LC-MS/MS virus analysis
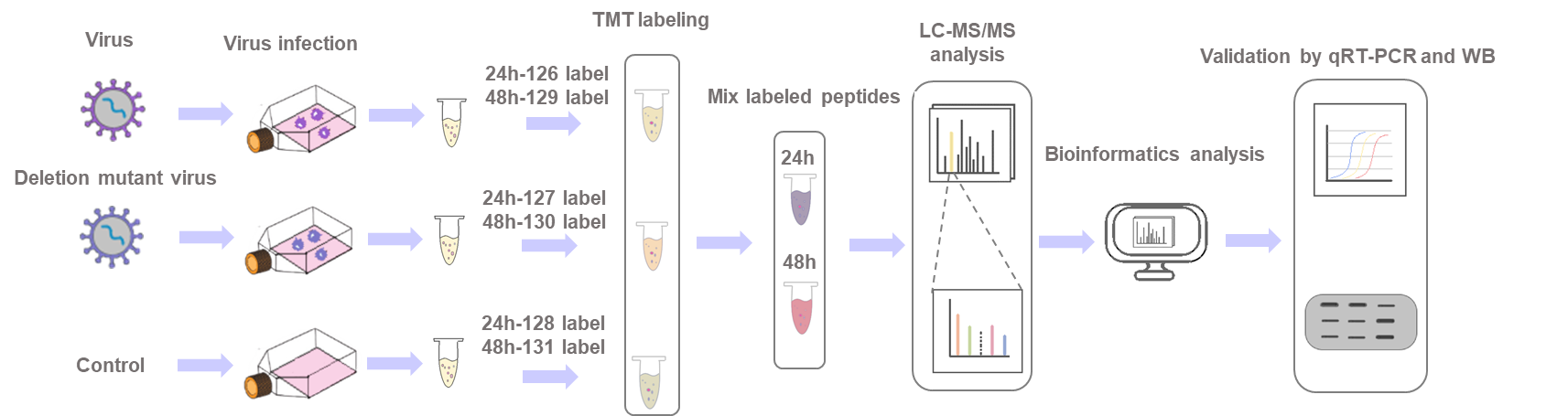
Service offering
Our relative quantitation platform is based on Thermo Scientific™ Orbitrap Fusion™ Lumos™ Tribrid™ mass spectrometer. Compared to other instrumentation, the instrument is capable of providing more accurate quantitation and higher protein identification quantity. We offer one-stop services, ranging from custom experimental design, virus infection, cell culture, and TMT identification and quantitation of proteins from multiplexed samples. Our workflow can be optimized according to customers' specific project needs for the highest quantitative accuracy as well as coverage depth.
Workflow of TMT quantitative proteomics

In the past decade, the entire proteomics workflows have made critical advances. Notably, the development of TMT 11plex isobaric tandem mass tag labeling combined with advancements in analytical MS leads to more accurate, high-throughput quantitative proteomics. If you are interested in our services, please don't hesitate to contact us. We are glad to cooperate with you and to witness your success!
Reference
- Duan, Z., et al. (2020). "TMT-based quantitative proteomics analysis reveals the attenuated replication mechanism of Newcastle disease virus caused by nuclear localization signal mutation in viral matrix protein." Virulence, 11(1), 607-635.
* For research use only.


