Virus Quantification Using Plaque Assay
Virus quantification is to count the number of viruses in a specific volume for the purpose of determining the virus concentration. This is a fundamental and critical step for both academic and commercial laboratories, involving research as well as production of viral antigens, antiviral agents and among others. Various biochemical assays and techniques have been developed for virus quantification, including traditional and modern methods. The former includes plaque assay, focus forming assay (FFA), transmission electron microscopy (TEM) and so on. And the latter involves tunable resistive pulse sensing (TRPS), flow cytometry, quantitative PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA). Different techniques afford different perspectives. With the help of our well-established technologies and skill scientists, Creative Proteomics is capable of quantifying viruses in complex biological samples. The plaque assay is the gold standard in quantification of infectious viral titer. Here, we provide an improved method, the automated plaque counting method, to meet the fast and accurate detection and quantification of viruses.
Plaque assays
Plaque assays were established in 1952 and first used as phage assays in plant biology. Later, the method was adapted to investigate the concentrations of viral samples. And nowadays, it has become a common type of infectivity assay by counting discrete "infectious centers". Although there are many new techniques for viral titration, this method still plays a crucial role in the quantification of infectious virus specimens due to its high stringency. In general, plaque assay is mainly comprised of four steps, including serial dilution of samples containing virus, specific cell culture (according to the type of virus), virus infection (including virus replicates and plaque generation), and plaque counting and analysis. Finally, results of plaque assays are usually demonstrated as plaque forming units (PFU) per volume of measure (mL). Since relying on viral growth kinetics and host cell culture, plaque assays generally take several days to complete. Besides, the method is labor intensive and susceptible to operation bias due to manual plaque counting.
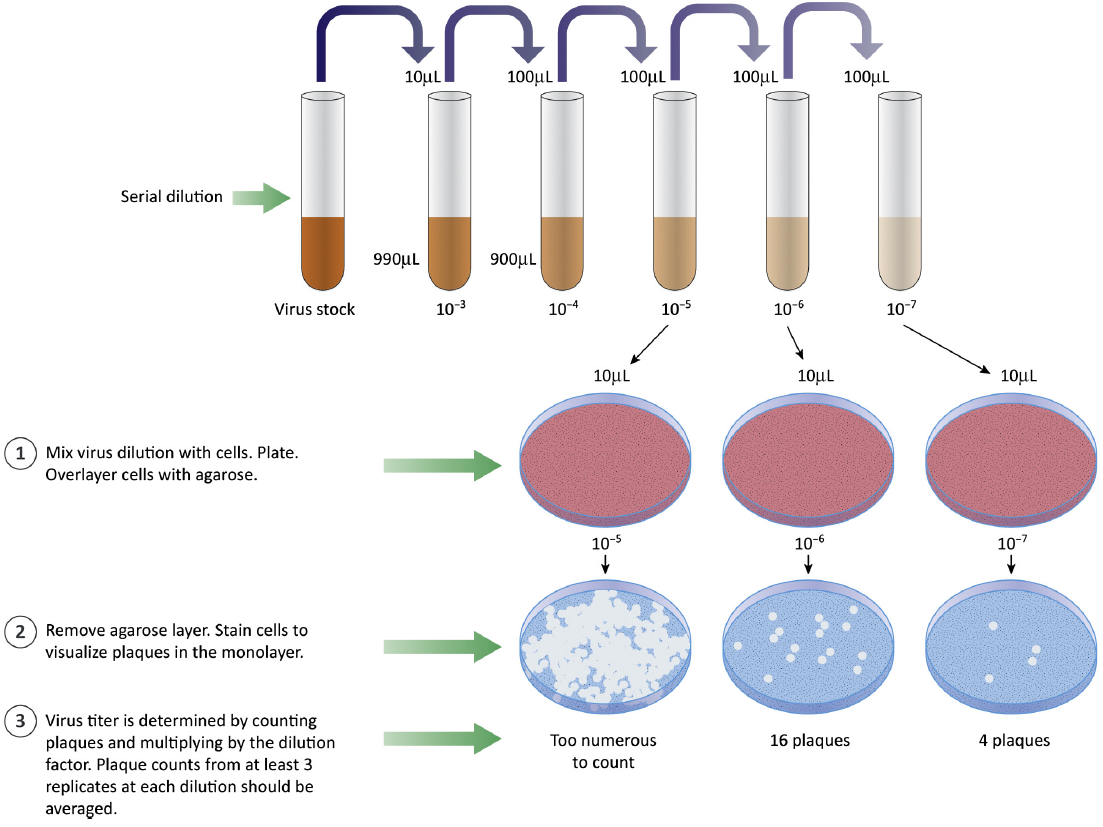 Fig 1. Plaque assays are used to quantify infectious virus particles. (Payne, S., 2017)
Fig 1. Plaque assays are used to quantify infectious virus particles. (Payne, S., 2017)
Service offering in Creative Proteomics
Plaque assays are the gold standards in investigation of infectious virions, which are direct and accurate. At present, it is a commonly used method and adapt to a number of different viruses. However, the method has disadvantages, including labor intensive, time consuming and so on. To meet the fast and accurate quantification of virus, we provide an improved plaque assay service based on the integration of fluorescently labeled plaques and automated imaging. The bottleneck of traditional plaque assay includes a manual counting method. On one hand, the plaques that must be large enough to be counted. On the other hand, there is analyst subjectivity. Our service is based on automated counting and analysis, thereby, analyst subjectivity can be eliminated. Besides, plaque counting time can also be significantly reduced, which is benefited from our system applied 96-well plates supporting a high volume of samples detection.
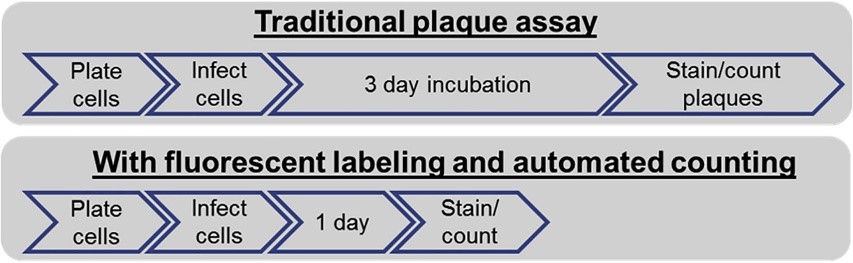 Fig 2. Flow Diagrams of Traditional and Improved Plaque Assay. (Masci, A. L., et al., 2019)
Fig 2. Flow Diagrams of Traditional and Improved Plaque Assay. (Masci, A. L., et al., 2019)
The advantages of our service
- A relatively fast virus detection/ quantification
- More reliable virus quantification by improved data capture
- Advanced instruments and highly experienced analysts
- Documentation of results, and traceability
With the help of automated counting and a computer algorithm for counting plaques in improved plaque assays, Creative Proteomics is capable of supporting virus detection and quantification in a relatively fast and sensitive manner. We are committed to providing customized and optimal strategies for each specific case. We are happy to cooperate with our customers from academic or commercial laboratories. If you have any doubts about our service, please don't hesitate to contact us for more details.
References
- Masci, A. L., et al. (2019). "Integration of fluorescence detection and image-based automated counting increases speed, sensitivity, and robustness of plaque assays." Molecular Therapy-Methods & Clinical Development, 14, 270-274.
- Payne, S. (2017). "Methods to study viruses." Viruses, 37.
* For research use only.

 Fig 1. Plaque assays are used to quantify infectious virus particles. (Payne, S., 2017)
Fig 1. Plaque assays are used to quantify infectious virus particles. (Payne, S., 2017) Fig 2. Flow Diagrams of Traditional and Improved Plaque Assay. (Masci, A. L., et al., 2019)
Fig 2. Flow Diagrams of Traditional and Improved Plaque Assay. (Masci, A. L., et al., 2019)