
- Home
- PTMs Proteomics
- 2-Hydroxyisobutyrylation Quantification Service
Acylations are pivotal regulatory mechanisms in a plethora of biological processes, influencing everything from transcriptional regulation to signal transduction. Dysregulation of acylations has been associated with various diseases. Acylations constitute a class of modifications involving the addition of short-chain acyl groups to the amino groups of lysine residues. Apart from extensively studied lysine 2-hydroxyisobutyrylation (Khib) modification, ten novel lysine acylations have recently been identified on both histone and non-histone proteins. These include lysine propionylation, butyrylation, 2-hydroxyisobutyrylation, succinylation, glutarylation, malonylation, β-alanylation, 3-hydroxybutyrylation, benzoylation, and lactylation. These translated modifications exhibit distinct structural characteristics, resulting in diverse functional impacts on proteins. These acylations are primarily regulated through different acyl-CoA metabolism pathways and are catalyzed by lysine acyltransferases that transfer acyl groups onto lysine side-chain amino groups.
In 2014, Lunzhi Dai and colleagues first reported lysine 2-hydroxyisobutyrylation (Khib) as a novel histone modification. They identified 63 Khib modification sites in human and mouse histones. Notably, the genomic distribution of Khib in histones during male germ cell differentiation differed from that of histone acetylation (Kac) or histone crotonylation (Kcr). Beyond histones, literature reports indicate that Khib is an evolutionarily conserved modification, widely expressed in a variety of species across prokaryotes and eukaryotes, affecting both histone and non-histone proteins. This emerging modification can influence chromatin structure and epigenetic pathways, potentially playing crucial roles in various cellular pathophysiological processes.
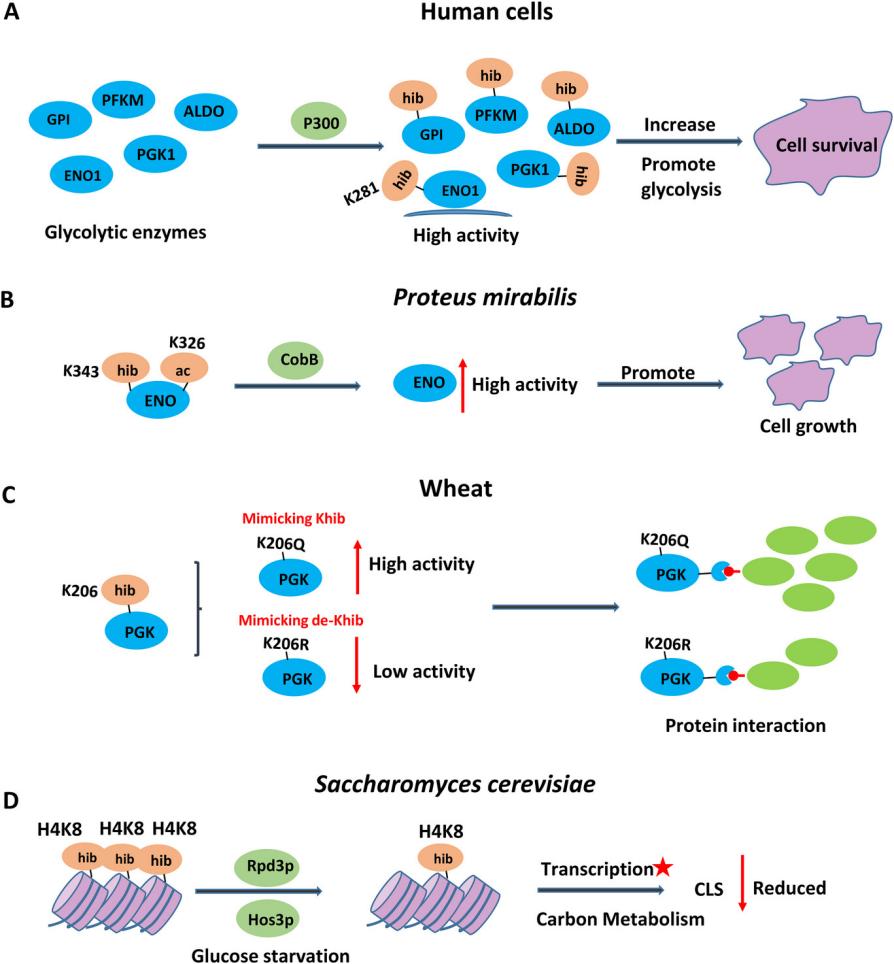 Functions of lysine 2‐hydroxyisobutyrylation[2]
Functions of lysine 2‐hydroxyisobutyrylation[2]
Creative Proteomics, a leader in protein and post-translational modification analysis, offers comprehensive solutions for both conventional proteomics and modification proteomics research, particularly focusing on novel protein post-translational modifications.
1. Protein Extraction and Trypsin Digestion
Cell lysates were prepared, and proteins were reduced, alkylated, and digested with trypsin. The resulting proteolytic peptides were purified and prepared for further analysis.
2. Immunoaffinity Enrichment
Khib peptides were enriched using pan anti-Khib beads, followed by washing and elution steps to isolate Khib-containing peptides.
3. LC-MS/MS Analysis
Enriched Khib peptides were analyzed using a high-resolution mass spectrometer. Peptides were separated by reversed-phase HPLC and analyzed in data-dependent acquisition mode, with MS/MS fragmentation.
4. Database Search and Data Filtering
The obtained MS/MS data were searched against a human protein database using MaxQuant and Andromeda search engine. Specific modification sites were identified and filtered based on quality criteria.
5. Bioinformatics Analysis
Pathway analysis was conducted to elucidate the functional roles of Khib substrates and establish Protein-protein interaction networks to explore potential connections to various biological processes and diseases.
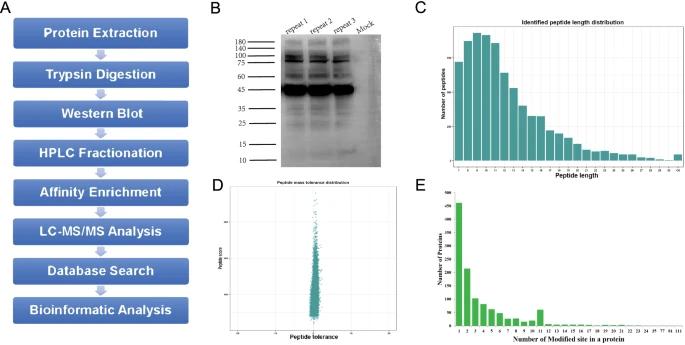 Identification of 2-hydroxyisobutyrylated proteins[3]
Identification of 2-hydroxyisobutyrylated proteins[3]
In the realm of protein post-translational modification (PTM) analysis, our services offer distinct advantages that cater to the diverse needs of researchers and scientists. These advantages are a testament to our commitment to providing comprehensive and reliable PTM analysis solutions.
Extensive Database Facilitating Unprecedented Protein Identification
Our capabilities extend to the identification of a substantial number of proteins, with the capacity to analyze in excess of 4000 proteins. This expansive database alleviates concerns regarding the scope of your protein identification requirements.
High-Resolution Mass Spectrometry Ensuring Robust Outcomes
We utilize cutting-edge high-resolution mass spectrometers, including Thermo Orbitrap Fusion Lumos, Thermo QE Plus, Thermo Q Exactive HF, Thermo Q Exactive HF-X, Thermo Orbitrap Exploris 480, and tims TOF Pro. These state-of-the-art instruments guarantee the precision of data and the detection of proteins. High-resolution mass spectrometry is imperative for delivering reliable and accurate results.
Comprehensive Array of Analysis Services
Our services encompass a wide spectrum of analysis options, offering a selection of over 40 analysis content choices. This comprehensive range of services equips you with the necessary tools and insights to effectively explore and visualize the results of protein quantification analysis.
Expedited Results Delivery, Eliminating Anxious Delays
We recognize the significance of time in research endeavors. Therefore, we streamline our experimental protocols to reduce project timelines, ensuring prompt delivery of your results. Bid farewell to anxious waiting and welcome enhanced efficiency.
Our PTM analysis services are flexible and adaptable to a wide range of sample types, including:
Animal Tissues: We handle both standard tissues (e.g., brain, heart, liver, kidney, muscle) and tougher tissues (e.g., skin, blood vessels, cartilage, hair).
Plant Tissues: Our services accommodate soft plant tissues (e.g., leaves, flowers, herbs, algae, ferns) as well as hard plant tissues (e.g., tree roots, bark, branches, fruits, seeds).
Cellular Samples: We specialize in the analysis of cellular samples.
Microorganisms: Our expertise extends to common bacteria, fungal cells, and precipitated microbial cells.
Liquid Samples: Liquid samples such as plasma, serum, cerebrospinal fluid, lymphatic fluid, synovial fluid, puncture fluid, abdominal fluid, breast milk, among others, are within our scope.
Pure Proteins: We can analyze pure protein samples.
Secreted Proteins: Our services extend to secreted proteins analyzed using techniques such as TMT, DIA, and 4D-proteomics.
FFPE (Formalin-Fixed, Paraffin-Embedded) Samples: We offer analysis for FFPE samples using label-free, TMT, PRM, and 4D-proteomics methods.
IP/Pull Down Samples: We can analyze samples after immunoprecipitation or pull-down assays, utilizing techniques like TMT, DIA, and 4D-proteomics.
Our protein post-translational modification services are tailored to meet your diverse research needs. With an extensive database, high-resolution mass spectrometry, a wide range of analysis services, and swift results delivery, we empower researchers to explore and understand protein modifications comprehensively. Our commitment to providing efficient and reliable services ensures that your research endeavors are supported effectively.
References
Our products and services are for research use only.