
- Home
- PTMs Proteomics
- Proteomics Analysis of Amidation
Amidation, a pivotal biochemical process, involves the conversion of carboxylic acids into amides. This transformation is catalyzed by amidating enzymes, presenting a critical mechanism in the synthesis of biologically active compounds. The significance of amidation transcends basic biochemistry, reaching into pharmaceuticals, biotechnology, and numerous other industries.

In the field of biochemistry, amidation is critical because it provides a flexible framework for the synthesis of molecules with a wide range of uses. As a company devoted to protein research, Creative Proteomics has used cutting-edge analytical techniques to assist researchers in deciphering the intricacies of the amidation reaction. This has enabled the precise control needed by numerous industries that depend on this essential biochemical process.
The effect of amidation on the behaviour of antimicrobial peptides
Journal: Eur Biophys J
Published: 2016
Background
AMPs are integral to the innate immune system and exhibit therapeutic activity against various microorganisms. The study investigates how post-translational modifications, particularly carboxyamidation, impact the antimicrobial activity of these peptides. Amidation is believed to enhance helix stability at the membrane interface, influencing efficacy. Previous research has demonstrated that peptide structure, especially the termini, is crucial for membrane binding. Amidation is thought to increase helix stability, and studies have shown that non-amidated peptides may have reduced antibacterial activity due to decreased positive surface charge and structural perturbations. Despite various experimental techniques, such as NMR, CD, fluorescence, and lipid monolayer analysis, a detailed understanding of the amidation mechanism is lacking.
Molecular dynamics (MD) simulations provide molecular-level insight into the interaction of amidated AMPs with membranes. Researchers have used MD to show that amidated peptides interact more strongly with lipid bilayers, potentially forming stable pores. The present study focuses on aurein 2.6 and 3.1 peptides and employs MD and CD spectroscopy to investigate their membrane interactions. The research aims to provide a comprehensive understanding of how amidation influences the membrane interaction of AMPs, shedding light on potential applications in antimicrobial therapy.
Results
The study explores the impact of amidation on the structure and stability of aurein peptides, specifically focusing on aurein 2.6 and aurein 3.1 isoforms. The investigation employs both experimental and computational techniques to analyze the peptide-membrane interaction. Circular dichroism (CD) spectroscopy is utilized for secondary structure analysis in various environments. In phosphate-buffered saline (PBS), both aurein 2.6 and aurein 3.1 isoforms exhibit characteristics of a random coil structure. However, in a membrane environment (TFE, DMPC, and DMPS membranes), both peptides adopt an α-helical conformation, which is typical for cationic AMPs at a membrane-lipid interface. Amidation effects on helicity are observed in different membrane environments. In the presence of DMPC vesicles, amidation enhances the helical content of aurein 2.6, while for aurein 3.1, amidation has no significant effect. Conversely, in the presence of anionic DMPS vesicles, both amidated and non-amidated aureins show increased helical content, with aurein 2.6-CONH2 exhibiting higher helicity compared to aurein 2.6-COOH. Similarly, aurein 3.1-CONH2 displays higher helical content compared to aurein 3.1-COOH in the presence of DMPS vesicles (Figure 1).
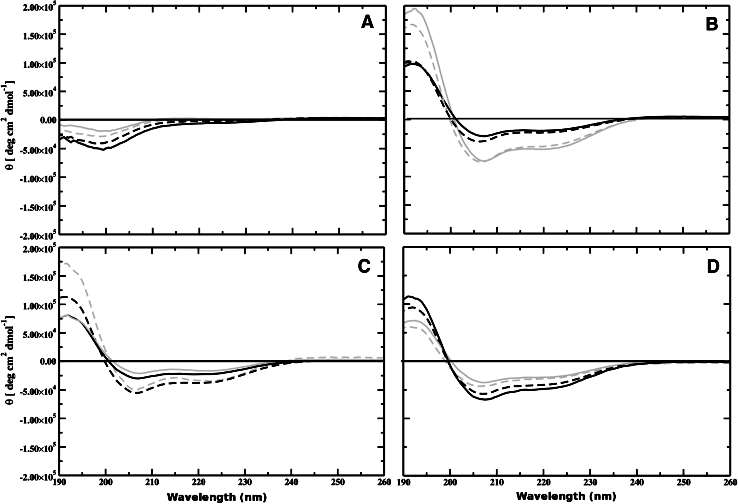 Figure 1
Figure 1
The study investigates the dynamics properties of aureins in the presence of a lipid bilayer using molecular dynamics (MD) simulations, with a particular focus on the role of the C-terminus in the peptide/lipid bilayer interaction. In both aurein peptides, the presence of NH2 at the C-terminus leads to deeper penetration into the head groups of the DMPC lipid bilayer. The MD simulations show the insertion of both N-terminus (in red) and C-terminus (in blue) into the DMPC bilayer. Non-amidated aureins, on the other hand, primarily interact with the lipid bilayer through the N-terminal residues, favoring a tilted insertion. Additionally, the insertion of aurein peptides into the DMPS bilayer is observed, indicating a distinct behavior compared to DMPC.
Amidation at the C-terminus results in different interactions. The NH2 group interacts with the head group of the DMPS lipid bilayer, enhancing the overall interaction of both N- and C-terminus with DMPS. Non-amidated aureins, in contrast, are situated in the water-lipid interface region, interacting with the head groups of the lipid bilayer. Different residues in amidated aureins are involved in the interaction with the lipid bilayer, influencing the penetration mechanism. The NH2 at the C-terminus is influenced by the presence of PO4 groups in DMPC and CO2 in the DMPS lipid bilayer. In the second set of simulations, when the peptides are inside the lipid bilayer, the presence of NH2 at the C-terminus highlights interactions with the head groups of the DMPC lipid bilayer. However, in the case of aurein 2.6-COOH, the C-terminus interacts with the inner part of the head groups of the DMPC lipid bilayer, showing increased mobility of the peptides inside the hydrophobic core of the phospholipid bilayer. The peptides maintain a stable secondary structure inside the lipid bilayer after 200 ns of simulation (Figure 2).
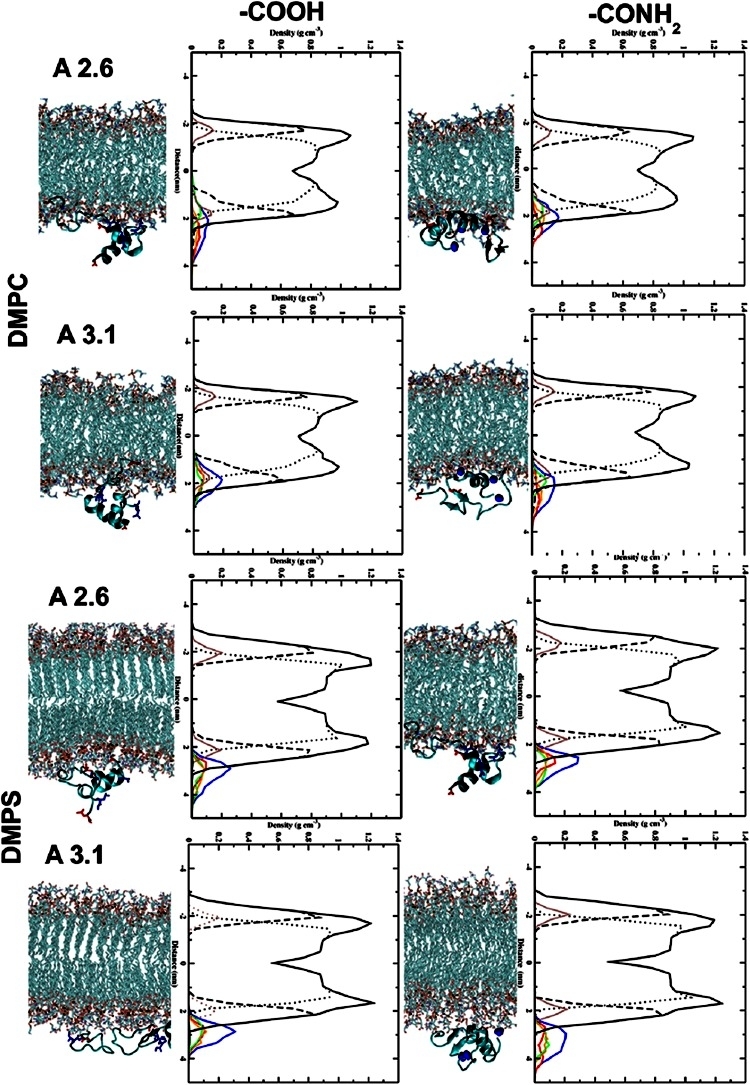 Figure 2
Figure 2
Conclusion
The study investigated the antimicrobial peptides (AMPs) Aurein 2.6-COOH and Aurein 3.1-COOH, comparing them with their naturally occurring C-terminally amidated analogues. Circular dichroism (CD) and molecular dynamic (MD) simulations were employed to analyze the impact of amidation on the interaction of these peptides with lipid bilayers. The findings indicated that both peptide analogues primarily exhibited a random coil structure with minimal α-helical content in solution (<30%). However, in the presence of a lipid bilayer, the peptides formed stable α-helical structures. Generally, amidated analogues showed a higher tendency than non-amidated peptides to adopt an α-helical structure. MD simulations predicted that Aurein 2.6-COOH and Aurein 3.1-COOH destabilized lipid bilayers and exhibited angled penetration in specific lipid environments. Additionally, the study revealed that amidated versions of these peptides formed a helix oriented horizontally to the plane of an asymmetric interface.
Reference
Our products and services are for research use only.