
- Home
- PTMs Proteomics
- Lipidation
- Proteomics Analysis of N-myristoylation
As an ubiquitous class of protein lipidation across eukaryotes and viruses, N-myristoylation has become a hot topic in protein modification research in the past decade. There is growing evidence for the importance of protein N -myristoylation in regulating cellular signaling pathways in a wide range of biological processes. Based on our advanced platform, Creative Proteomics is proud to offer protein N-myristoylation analysis service. Our methods include state-of-the-art mass spectrometry (MS)-based proteomics, metabolic labeling, western blotting (WB), Immunoprecipitation, immunofluorescence analysis, and so on. We are committed to providing our clients with flexible, customized, and affordable protein N-myristoylation identification and characterization.
Protein N-myristoylation is a cotranslational lipidic modification of many eukaryotic and viral proteins, which is regulated by the ubiquitous eukaryotic enzyme, N-myristoyl transferase (NMT). Precisely, NMT using myristoyl-coenzyme A (CoA) as a substrate catalyzes the myristoylation process of myristic acid attached to the N-terminus, thus increasing protein-protein interactions. Myristoylation usually occurs on newly synthesized peptides after the initial methionine is cleaved by methionine aminopeptidase. It has also been reported that NMT-mediated post-translational modifications (PTMs) of proteins occur after hydrolytic cleavage of proteins containing N-terminal glycine residues. N-myristoylation plays vital roles in cellular signaling, protein-protein interaction, and plasma membrane system. Increasing evidence has shown that protein N-myristoylation is involved in a wide range of biological processes, including carcinogenesis, immune function, hematopoiesis, and viral infections. Therefore, N-myristoylation and NMT have been proposed as attractive drug targets for several pathogens.
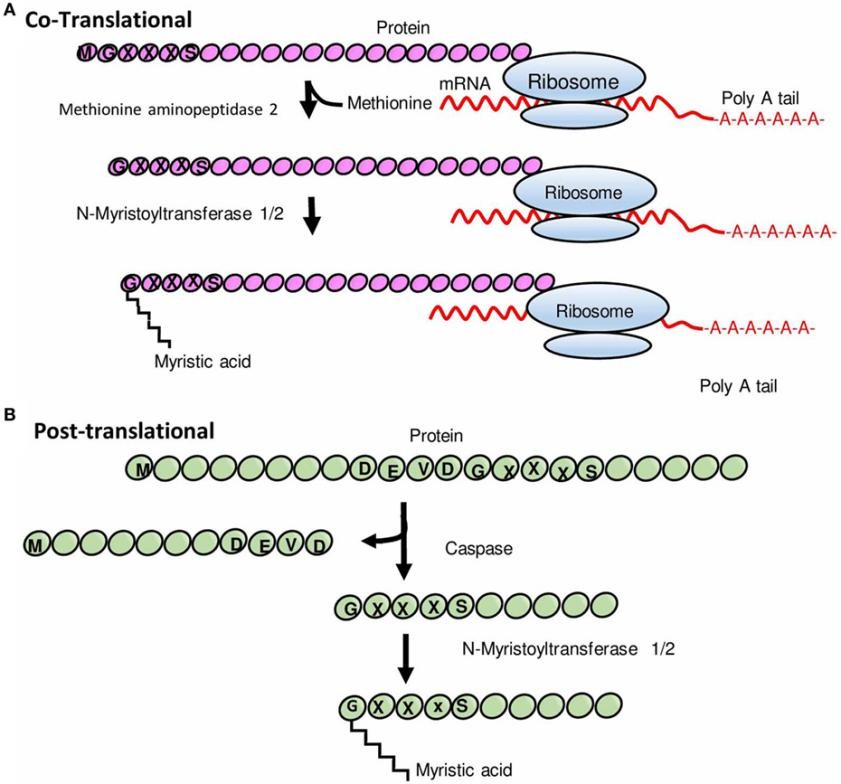 Fig. 1 Schematic representation of N-myristoylation of proteins. (A) Cotranslational protein N-myristoylation. (B) Posttranslational protein N-myristoylation. (Udenwobele, Daniel Ikenna, et al., 2017)
Fig. 1 Schematic representation of N-myristoylation of proteins. (A) Cotranslational protein N-myristoylation. (B) Posttranslational protein N-myristoylation. (Udenwobele, Daniel Ikenna, et al., 2017)
Creative Proteomics is the leading custom service provider in PTM proteomics analysis. We strive to be a guide and assistant in protein N-myristoylation research for our customers worldwide. According to the specific requirements of our customers, we provide the optimal strategies and strict workflow for N-myristoylation analysis. The prediction of protein N-myristoylation can be performed using advanced prediction procedures. To visualize the extent of myristoylation in mammalian cells and protozoan parasites, metabolic labeling approaches are commonly used. In addition, we use MS-based proteomics combined with validated chemical tools for the confident identification and characterization of labeled proteins. Specifically, our service includes the following lists:
Protein N-myristoylation has been shown to be an important evolutionarily conserved modification of proteins implicated in different physiological processes. If you are interested in our protein N-myristoylation analysis services, please contact us. All you need to do is place an order and send a sample, and we provide a one-stop service.
Reference
Our products and services are for research use only.