
- Home
- PTMs Proteomics
- Proteomics Analysis of Cysteinylation
Cysteinylation, a post-translational modification (PTM) involving the covalent attachment of cysteine residues to various biomolecules, has emerged as a critical player in the realm of cellular regulation. This complex process affects multiple cellular pathways and adds to the diversity of protein functions. Deciphering the subtleties of cysteinylation is essential to understanding the intricate relationships between cellular signaling and function. Continued advancements in analytical techniques, coupled with integrative omics approaches, promise a more comprehensive understanding of cysteinylation. Exploring its dynamic nature and functional consequences will undoubtedly uncover novel aspects of cellular regulation.
Accurate detection and characterization of cysteinylation are pivotal for dissecting its role in cellular processes. Several sophisticated analytical methods have been developed to scrutinize cysteinylation events at the molecular level.
Cysteinylation plays a pivotal role in diverse cellular processes, influencing protein structure, function, and interactions. Understanding its applications provides a foundation for deciphering its functional implications.
Cysteinylation stands as a key player in the intricate landscape of post-translational modifications. Creative Proteomics utilizes various advanced technologies and analytical methods, insights into its diverse applications, and exploration of cross-talk with other PTMs position cysteinylation as a focal point for unraveling the complexities of cellular regulation. As research in this field advances, the potential for therapeutic interventions and a deeper understanding of disease mechanisms continues to expand, marking cysteinylation as a cornerstone in the biological sciences.
Case Study
Identification and quantitation of hinge cysteinylation on endogenous IgG2 antibodies from human serum
Journal: MAbs
Published: 2020
Background
Cysteinylation involves the addition of a terminal cysteine onto a cysteine residue in a protein via a disulfide bond. In therapeutic mAbs, cysteinylation may occur in the hinge regions, as shown in the case of recombinant IgG2 antibodies. The article emphasizes the need to control product quality attributes related to cysteinylation, as changes in mAbs' biochemical and biophysical properties can impact their bioactivity, pharmacokinetics, safety, and immunogenicity.
The study focuses on hinge cysteinylation in IgG2 antibodies and highlights that little information is available on the safety or immunogenicity risks associated with this modification. The authors propose an alternative to clinical trials by examining whether hinge cysteinylation is present in endogenous human IgG2s. The study involves selectively enriching IgG2s from human serum, using targeted mass spectrometry to quantify endogenous levels of hinge cysteinylation. The findings indicate the first reported evidence of quantifiable levels of cysteinylation in the hinge region of endogenous human IgG2s.
Results
The study focuses on the doubly cysteinylated IgG2-A in recombinant IgG2 mAb1. Two processes, Y and Z, were used as positive and negative controls. The characterization of hinge cysteinylation in mAb1-Y involved a bottom-up examination of Lys-C dipeptides corresponding to doubly cysteinylated and classically linked IgG2-A forms. Masses consistent with a 5+ charge state of the doubly cysteinylated and classically linked hinge Lys-C dipeptides were observed in mAb1-Y single ion monitoring (SIM) experiments. Extracted ion chromatograms (XIC) indicated that the doubly cysteinylated dipeptide eluted approximately 1 minute earlier than the classically linked dipeptide, suggesting greater hydrophilicity due to the presence of two terminal cysteine residues (Figure 1).
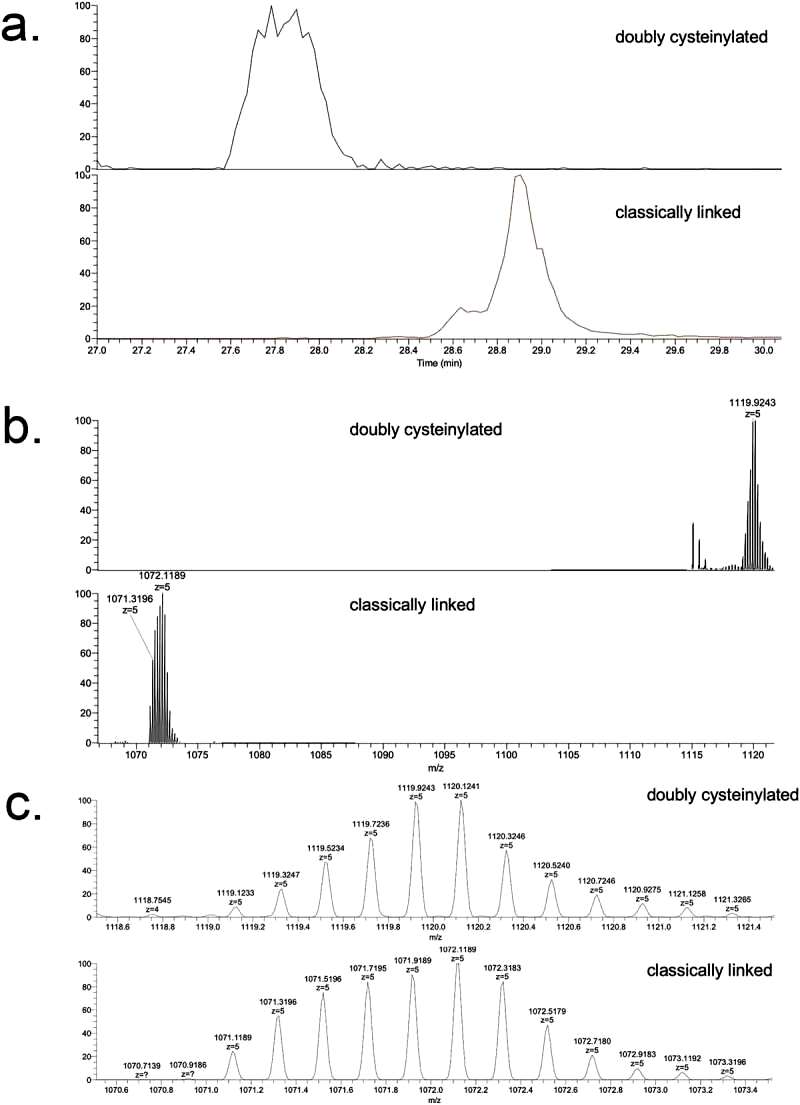 Figure 1
Figure 1
The identifications of doubly cysteinylated IgG2-A in recombinant IgG2 mAb1 were verified using tandem mass spectra from parallel reaction monitoring (PRM) experiments with collision-induced dissociation (CID) fragmentation. The observed y-product ion series confirmed the majority of the backbone amino acid sequence, while specific b8 and b10-product ions distinguished the doubly cysteinylated form from the classically linked hinge dipeptide. The mass difference between the b8-product ions corresponding to the doubly cysteinylated and classically linked dipeptides aligned with the expected mass increase due to two additional terminal cysteines.
The b8 and b10-product ions indicated that cysteinylation occurs on one of the four hinge interchain disulfide bonds, but further localization was not possible due to the lack of additional fragmentation ions. The study quantified the relative abundance of the doubly cysteinylated IgG2-A form in mAb1-Y, determining it to be 4.3%. Peak splitting in the XIC peak corresponding to the doubly cysteinylated dipeptide was observed, though the reason for this heterogeneity remains unclear. In contrast, mAb1-Z had a doubly cysteinylated IgG2-A form below the detection limit in the analysis (Figure 2).
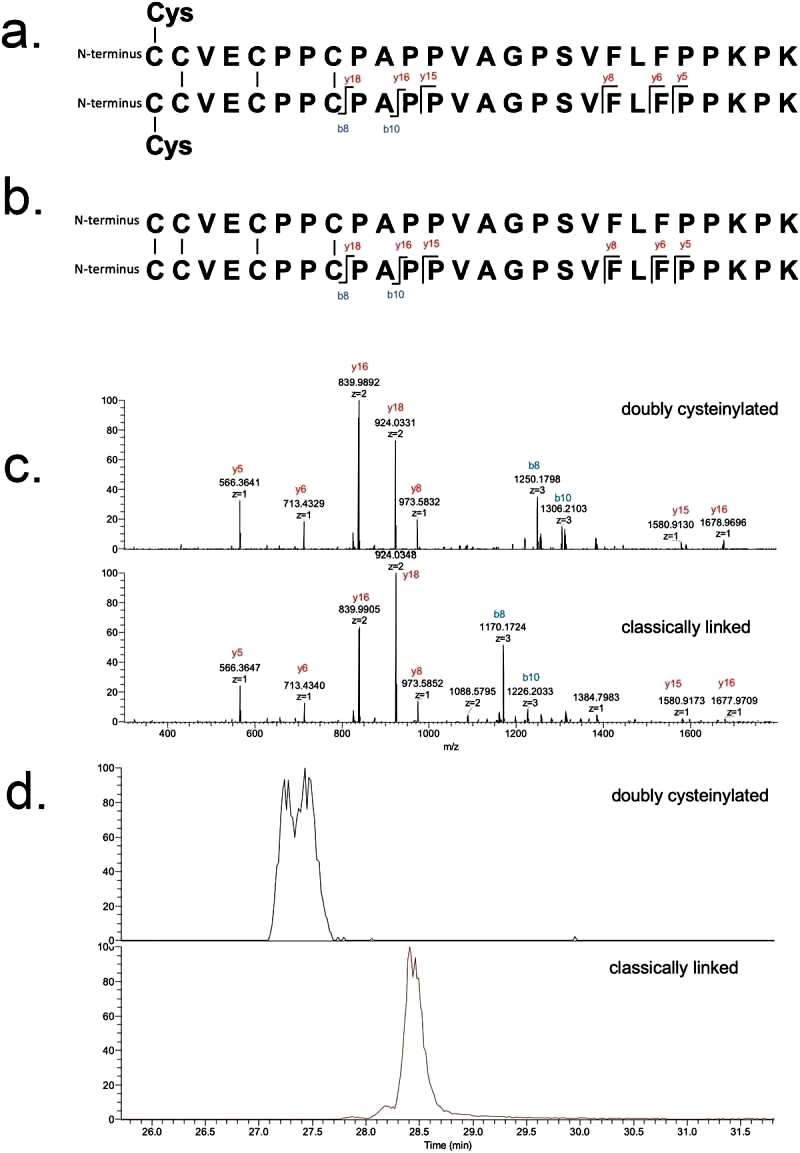 Figure 2
Figure 2
The anti-IgG2 immunoaffinity purification method employed in the study was validated by confirming IgG2 enrichment. A cocktail sample containing recombinant IgG1, IgG2, and IgG4 underwent Protein G pulldowns and anti-IgG2 pulldowns. While all three IgG subclasses were present in the Protein G pulldowns, only IgG2 was found in the anti-IgG2 pulldowns, demonstrating the specificity of the immunoaffinity purification method (Figure 3). To further validate the method, a control sample with 0.3% doubly cysteinylated IgG2-A was subjected to anti-IgG2 immunoaffinity purification, and the %2Cys levels remained consistent before and after purification, indicating no bias in %2Cys quantitation due to the pulldown. Repeatability analysis of the 0.3% doubly cysteinylated IgG2-A control sample showed a signal-to-noise (S/N) ratio of 10, suggesting a limit of quantitation (LOQ) of <0.3% and a limit of detection (LOD) of <0.1% doubly cysteinylated IgG2-A using parallel reaction monitoring (PRM) analysis.
Additionally, a set of IgG2 standards with % doubly cysteinylated IgG2-A levels ranging from 0.3% to 1.7% were prepared, and their %2Cys levels were measured using PRM analysis. The results demonstrated a linear relationship between observed %2Cys and theoretical %2Cys, with an R2 value exceeding 0.99. These experiments focused on a narrow range near the limit of quantitation, anticipating that endogenous IgG2s, if present, would likely exhibit low levels of %2Cys (Figure 4).
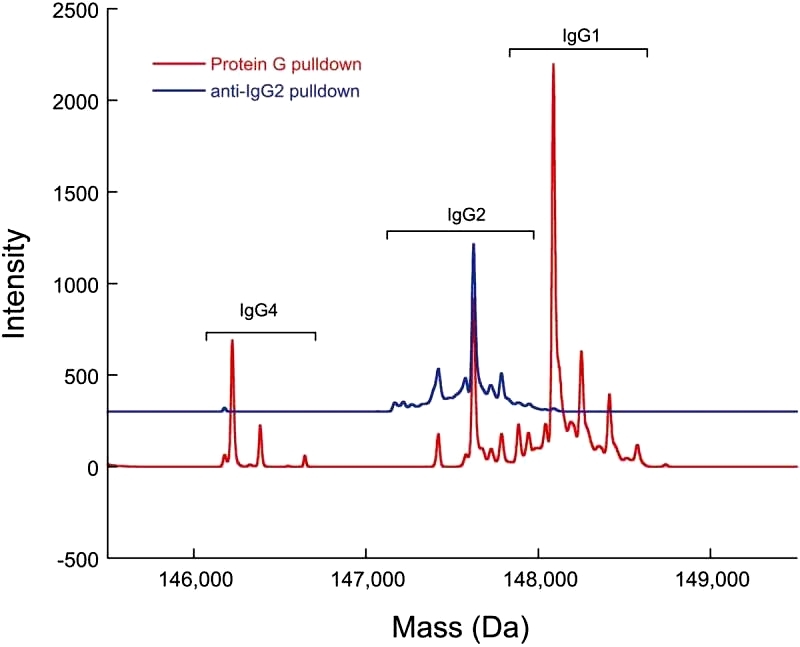 Figure 3
Figure 3
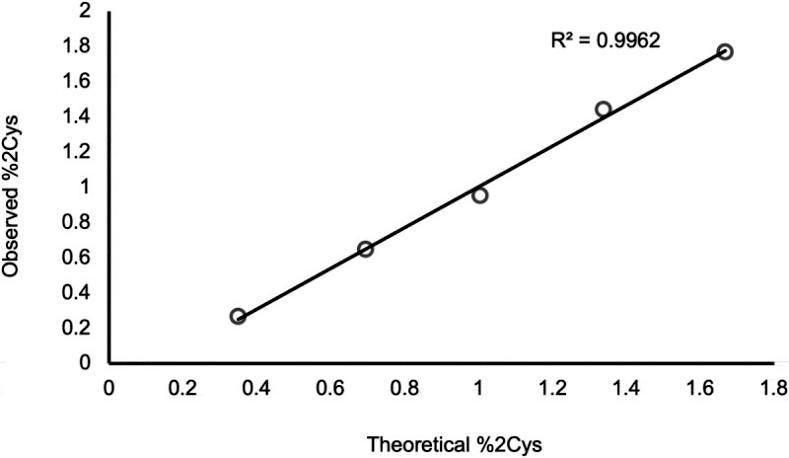 Figure 4
Figure 4
The study extended its investigation to endogenous IgG2s affinity purified from healthy human serum using the same method applied to recombinant mAb1. Single ion monitoring (SIM) experiments for endogenous IgG2s lacked specificity due to interference from a species carried over from the complex biological matrix. However, parallel reaction monitoring (PRM) experiments provided additional specificity, generating high-quality spectra and expected extracted ion chromatogram (XIC) peak retention times. The PRM experiments revealed compelling evidence for the presence of doubly cysteinylated IgG2-A in human serum. This modification was consistently identified in all 10 analyzed human serum samples, with levels ranging from 0.5% to 1.3% and averaging 0.8 ± 0.3%. These findings suggest that healthy individuals are continuously exposed to low but detectable levels of IgG2 hinge cysteinylation (Figure 5).
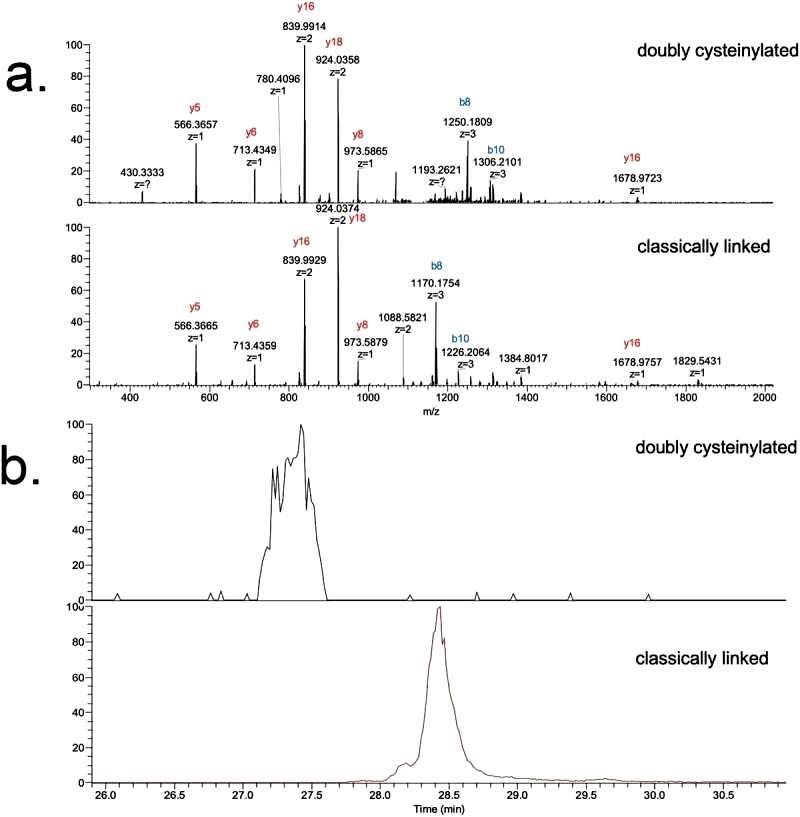 Figure 5
Figure 5
Conclusion
The study focuses on cysteinylation, a post-translational modification (PTM) occurring when a cysteine residue forms a disulfide bond with a terminal cysteine molecule on a protein. While this PTM has been observed in the hinge region of therapeutic IgG2 antibodies, its impact on safety and immunogenicity remains unclear. The research aims to characterize both recombinant and endogenous IgG2 antibodies, quantifying hinge cysteinylation levels. Using anti-IgG2 immunopurification of human serum and targeted mass spectrometry techniques, the study identifies and quantifies hinge cysteinylation in endogenous IgG2 antibodies from healthy human serum. Results show that all tested samples (N = 10) contain quantifiable levels of hinge cysteinylation (0.8 ± 0.3%) in their endogenous human IgG2s (IgG2-A isoform). The findings suggest that hinge cysteinylation in therapeutic IgG2s, up to a certain level, is well tolerated in humans, with minimal safety or immunogenicity risks.
Reference
Our products and services are for research use only.