
- Home
- PTMs Proteomics
- Peptide Enrichment for MS-Based Analysis
- Strong Anion/Cation Exchange Chromatography (SAX, SCX)
Mass spectrometry (MS)-based techniques are powerful tools used in the identification and quantitation of modified proteins and their post-translational modifications (PTMs). Ion-exchange chromatography (IEC), including strong anion/cation exchange (SAX, SCX), is a common method for protein and peptide separation. Creative Proteomics is a leading custom service provider in PTM proteomics analysis. Here, our expert team is providing SAX and SCX chromatography for the fractionation of PTMs to facilitate the project process for our customers.
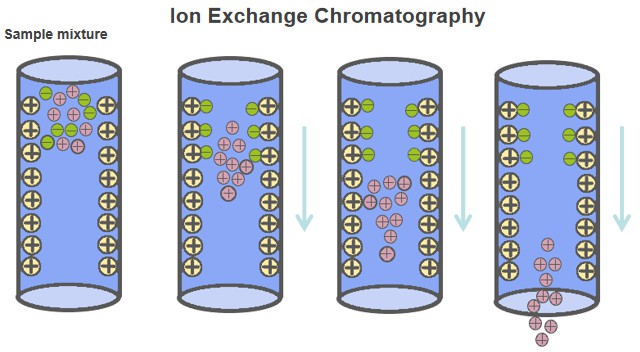
IEC is a relatively straightforward and efficient charge-based strategy that reduces the complexity of biological samples and enriches modified peptides based on the interactions between negatively charged groups and SCX or SAX substrates. SCX chromatography is a commonly used fractionation strategy for the enrichment of phosphorylated, and acetylated, and methylated peptides. After trypsin digestion, at low pH, the N-terminal amino and C-terminal lysine/arginine residues of most non-phosphopeptides are protonated and have a net charge of 2+. Since the presence of a single unit of a negatively charged phosphate group, the monophosphopeptides have a charge state of only 1+, which means that the net charge of a phosphopeptide decreases by one unit for each phosphate group added. Due to the SCX column containing a negatively charged stationary phase, the phosphorylated peptide will co-elute earlier than the unmodified peptide. As a complement to SCX, SAX chromatography was introduced, which tends to retain the negatively charged phosphopeptides more effectively than non-phosphorylated peptides. This method has shown better selectivity for multiply phosphorylated peptides.
When studying PTMs, an additional pre-fractionation step is required if the peptide sample is too complex or the dynamic range is too wide. After peptide extraction and prior to LC-MS/MS analysis, we apply high-performance liquid chromatography (HPLC) methods to facilitate peptide separation to reduce sample complexity and expand detection and coverage, including SCX and SAX chromatography. We are dedicated to offering a perfect solution for the separation and enrichment of modified peptides. All you need to do is tell us your experimental objectives and send us your samples, and we will take care of all the follow-ups of your project, complete with long sample preparation and analysis as well as complex data processing. During the chromatographic separation process, we consider and select the appropriate fractionation methods, column resins, and pH gradients according to the specific samples of our customers. For the subsequent MS analysis, preliminary studies are recommended to determine the appropriate charge and mass ranges for the endogenous peptides of interest.

For clients wishing to quantify protein PTMs by MS analysis, Creative Proteomics now offers a wide range of separation and enrichment methods and services. We are employing an application-oriented development strategy with a particular focus on experimental design and high-quality services. Feel free to contact us if you are interested in our modified enrichment services.
References
Our products and services are for research use only.