
- Home
- PTMs Proteomics
- Lipidation
- Proteomics Analysis of Prenylation
Protein prenylation is a C-terminal lipid modification and has been found to play an important role in protein-membrane and protein-protein interactions, and signal transduction. As a professional PTM analysis service provider, Creative Proteomics offers various post-translational modification (PTM) analysis services to help our customers to accelerate research progress and obtain better results. Here, we also offer a protein prenylation analysis service. With an advanced platform and strict workflow, we are able to analyze protein prenylation in various complex samples to meet your requirements.
Protein prenylation, also called geranylgeranylation, is an irreversible PTM that refers to the process of attaching two types of isoprenoid groups (a farnesyl or geranylgeranyl isoprenoid) to a C-terminal cysteine motif via farnesyl transferase, geranylgeranyl transferase, and other cellular prenyltransferase enzymes. This modification enhances the hydrophobicity and promotes membrane anchoring of Rho proteins, which is considered essential for proper subcellular targeting, effector binding, GTP loading and activation. As an evolutionarily conserved modification, prenylation has attracted extensive research interest for several reasons. It is reported that prenylated proteins have been linked to diseases such as cancer, inflammation and premature aging. Therefore, enzymes that catalyze reactions to generate mature prenylated proteins are being investigated as drug targets for related diseases. In addition, prenylated proteins play an important role in plant developmental processes and stress response because the incorporation of hydrophobic prenyl chains (mainly farnesyl or geranyl alcohol) makes hydrophilic proteins into peripheral lipid membrane proteins.
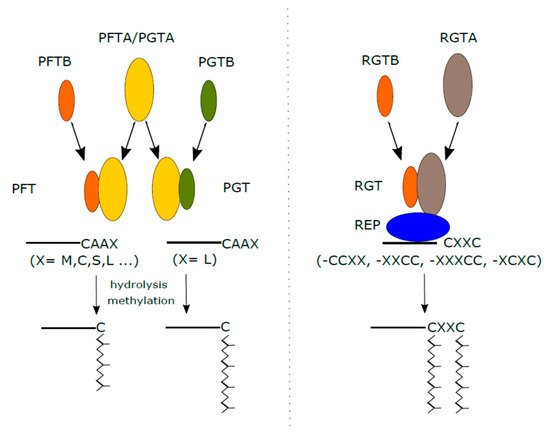 Fig. 1 Protein prenylation in plants. (Hála,
Michal, and Viktor Žárský. 2019)
Fig. 1 Protein prenylation in plants. (Hála,
Michal, and Viktor Žárský. 2019)
Prenylated proteins are well conserved across species, highlighting the biological and evolutionary importance of this lipid modification regulation. Over the years, we have developed highly efficient MS-based proteomics technology for system-wide identification and quantification of PTMs, including protein prenylation. Since metabolic labeling has been widely used in the analysis of protein prenylation, our expert team uses metabolic labeling methods in combination with enrichment. The obtained prenylated proteins will be identified by subsequent trypsin digestion and LC-MS analysis. Based on a powerful and professional bioinformatics platform, we are capable of helping our clients achieve efficient and rapid prenylated protein identification and quantification. Our service consists of five major steps:
The identification of intracellular prenylated proteins provides insight into the cellular processes and the etiology of disease associated with prenylation, providing the basis for the development of new, necessary and highly valuable related drug targets. Creative Proteomics continues to improve our resources and expand our services, and is committed to providing high-quality services to accelerate our customer service projects. We have assisted and witnessed the success of many of our partners. In order to get more details about our prenylation analysis service, please contact us. We've got everything covered for your needs.
References
Our products and services are for research use only.