
- Home
- PTMs Proteomics
- Proteomics Analysis of Carbamylation
Biological research is paying more and more attention to carbamylation, a chemical alteration that happens when proteins react with urea or its derivatives non-enzymatically. This phenomenon, which affects protein structure, function, and stability, is essential to many physiological and pathological processes.
A carbamoyl group is covalently added to amino acid residues, primarily lysine residues in proteins, to cause carbamylation. The process involves isocyanic acid, a byproduct of the breakdown of urea, nucleophilically attacking the amino group. The proteome becomes more complex as a result of this chemical change, which produces stable carbamylated proteins.
Accurate detection and quantification of carbamylated proteins are paramount for understanding their biological implications. Several analytical methods have been developed to unravel the intricacies of carbamylation.
Understanding the implications of carbamylation has far-reaching consequences across diverse fields, from clinical research to drug development.
Previously thought to be a byproduct of urea metabolism, carbamylation is now a key participant in protein modification. By combining cutting-edge analytical techniques, Creative Proteomics paves the way for a thorough investigation and unveils the complex functions that carbamylation plays in both health and illness. The potential for novel therapeutic approaches and diagnostic advancements grows as we learn more about the molecular details of this change, pointing to a time when carbamylation will be recognized as a crucial component of biological complexity rather than merely a chemical reaction.
Protein carbamylation is a hallmark of aging
Journal: PANS
Published: 2016
Background
Aging is a gradual process determined by genetic and acquired factors. It includes chemical reactions known as non-enzymatic post-translational modifications (NEPTMs) such as glycosylation, oxidation, carbonylation and carbamoylation. Glycosylation is generally considered to make an important contribution to the aging process, and carbamoylation is a recently described NEPTM that is caused by the non-enzymatic binding of isocyanates produced by urea dissociation or myeloperoxidase-mediated thiocyanate catabolism to the free amino groups of proteins. This modification is considered to be an adverse reaction because it induces changes in protein and cellular properties.
To date nothing is known about histone carbamylation during aging, and to address this issue the authors assessed the rate of homocitrulline (i.e., ε-aminomethyllysine, HCit), the most typical carbamylation derivative products (CDPs) that can be specifically quantified, in the skin of mammals with different life expectancies.
Results
The rate of protein carbamylation in skin extracts was determined using the HCit quantification method. In all species, HCit concentrations increased significantly with age(Figure 1).
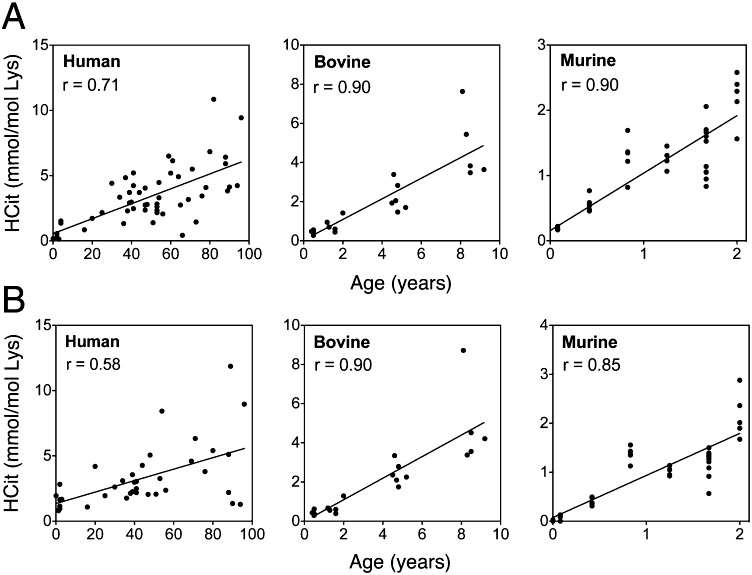 Figure 1
Figure 1
HCit content was determined by LC-MS/MS method, A graph shows the HCit content in total skin extracts and B graph shows the HCit content in skin type I collagen, which gradually increased with age in all studied species.
Immunohistochemical studies of skin samples from young and elderly individuals confirmed the increase in skin carbamylation intensity with age. For this purpose, the authors prepared and verified the specificity of an affinity-purified rabbit polyclonal antibody to HCit, and using this anti-Hcit specific antibody, the authors localized carbamoylated proteins to aged or young human skin sections. Faint fluorescence was observed in the skin of a 20-year-old human(Figure 2).
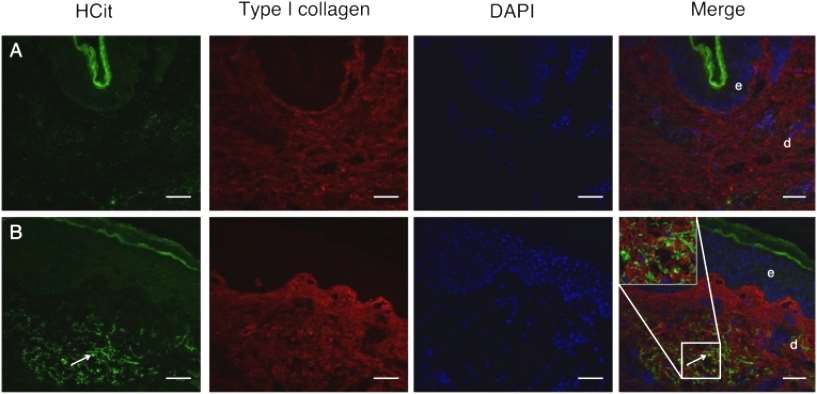 Figure 2
Figure 2
There was a significant correlation between the rate of HCit accumulation and life expectancy in total skin extracts and skin type I collagen.A graphs were calculated for each species for both nonlinear regressions (shaded area) and linear regressions (dashed line) of HCit concentration over time, and for better clarity these graphs focus on a 30-year cycle(Figure 3).
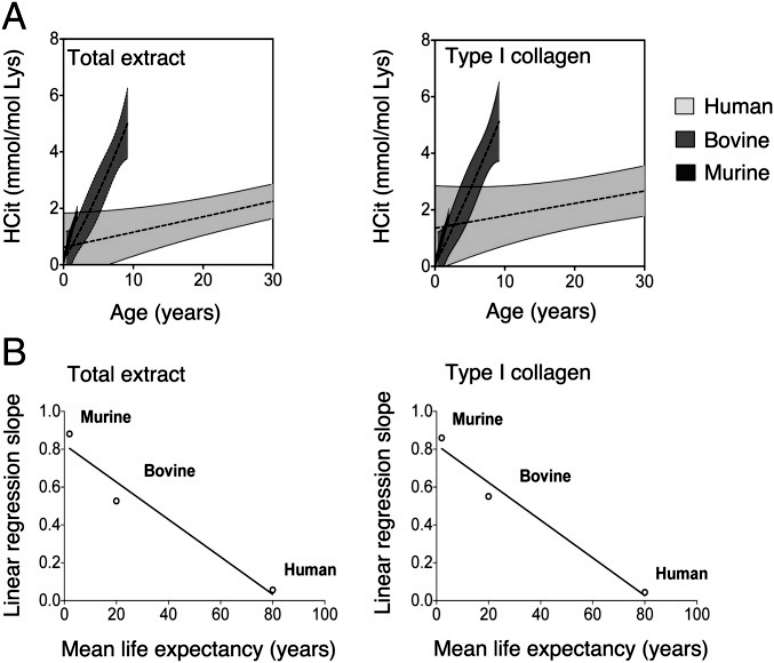 Figure 3
Figure 3
Conclusion
In this study, the authors assessed the carbamylation of three mammalian skin proteins with different lifespans and showed, for the first time, that carbamylation is an age-related process. Indeed, HCit increased 29-fold in mice from birth to old age, whereas in cattle and humans, it increased only 11.5-fold and 8.1-fold, respectively. However, despite the fact that HCit accumulates at the highest rate in mice, it reaches lower absolute levels at the end of life than in humans. Carbamoylation is a cumulative and time-dependent response, explaining the fact that species with longer lifespans exhibit higher levels of cutaneous HCit.
When examining the partial removal of carbamoylated proteins from organisms, it was found that plasma proteins are completely renewed due to their short half-life, whereas carbamoylated skin proteins are only partially removed from organisms, speculating that CDPs may be under homeostatic control. We can hypothesize that this decline is related to the shorter half-life of some carbamoylated proteins or to the elimination of intracellular CDPs through protein hydrolysis systems, such as the proteasome pathway or autophagy (which has been reported in the Proteasome-dependent degradation of intracellular carbamylated proteins).
Reference
Our products and services are for research use only.