
- Home
- PTMs Proteomics
- Proteomics Analysis of Cysteine-Redoxome Proteomics
Cysteine, a sulfur-containing amino acid, plays a pivotal role in cellular redox signaling. The burgeoning field of Cysteine-Redoxome Proteomics focuses on deciphering the intricate network of redox modifications involving cysteine residues within proteins.
Cysteine-redoxome proteomics analysis is important because it sheds light on the complex and dynamic cellular mechanisms involved in redox control. In many physiological and pathological situations, reactive oxygen species (ROS) and reactive nitrogen species (RNS) play a key role in redox signaling. Because cysteine residues in proteins are particularly susceptible to redox alterations, researchers can better understand the functional effects of these modifications by examining the Cysteine-redoxome proteomics. Deciphering the intricacies of redox signaling in health and illness necessitates the analysis of cystine-redoxome proteomics. It advances not just our knowledge of basic biological functions but also has applications in personalized medicine and treatment development.
![]()
Cysteine-redoxome proteomics stands as a transformative discipline in the realm of cellular redox signaling. Creative Proteomics provides advanced analytical methods to help researchers advance the understanding of the cysteine redoxome. As we continue to unravel the complexities of the cysteine redoxome, the potential for novel therapeutic targets and redox-responsive interventions becomes increasingly promising.
Global profiling of distinct cysteine redox forms reveals wide-ranging redox regulation in C.elegans
Journal: Nat Commun
Published: 2021
Background
Redox modification of cysteine plays an important role as a part of protein post-translational modification. In this thesis, the model organism Cryptomeria japonica was used as a carrier to explore the multiple redox forms of cysteine, revealing that cysteine redox can regulate various biological processes and pathways in the nematode, and finding that proteins upstream and downstream of the p38 MAPK pathway can mediate Cys-dependent antioxidant response and natural immune regulation.
Results
The authors applied the technique of quantification of protein sulfhydryl reactivity to wild-type L4 stage nematodes. Treatment with high (100 uM) and low (10 uM) doses of IPM probes detected reactivity at a total of 5258 cysteine sites, greatly extending the coverage of the cysteine proteome. The analysis suggests that there may be significant differences in the reactivity of cysteines in the same protein, and that the reactivity of cysteines can be used as an effective predictor of their function (Figure 1).
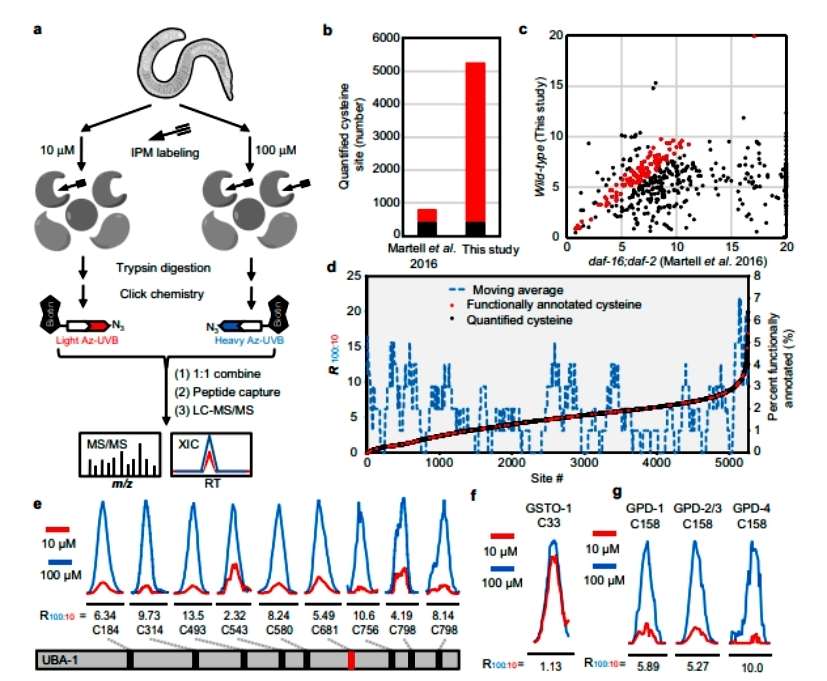 Figure 1
Figure 1
The authors used a redox proteomics approach based on "click chemistry" probes to accurately quantify the major cysteine redox forms in complex proteomes. The authors treated wild-type nematodes with 5 mM H2O2 for 5 min and labeled them with different probes. The results showed that 5453 Cys-SH sites were localized on 2864 proteins, 1521 Cys-SOH sites on 1049 proteins and 82 Cys-SO2H sites on 72 proteins. And more than 1500 protein Cys sites were found to be significantly changed after exogenous low concentration H2O2 treatment (Figure 2).
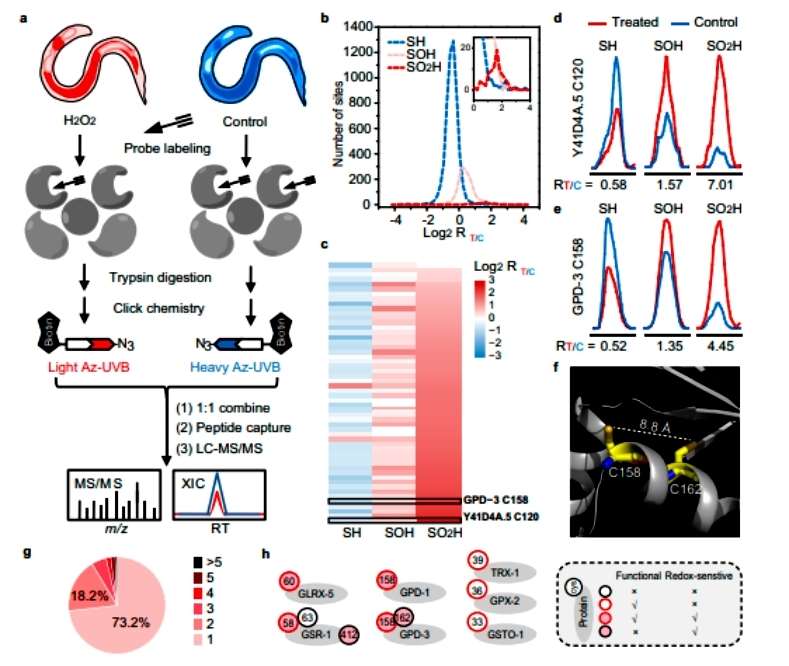 Figure 2
Figure 2
The authors found redox-modified proteins localized in different tissues and organs, which were subjected to GO and KEGG analysis. Bioinformatics analysis revealed that these oxidation-sensitive proteins are involved in numerous biological pathways. The focus was on whether SEK-1 and PMK-1 play a redox-regulatory role in p38 MAPK-mediated stress response and pathogen resistance (Figure 3). The authors constructed a point mutant nematode model using Cas9/CRISPR gene editing and found that both C173 of PMK-1 and C213 of SEK-1 are involved in oxidative stress-mediated activation of the p38MAPK pathway, and that this is critical for nematode resistance to pathogens (Figure 4).
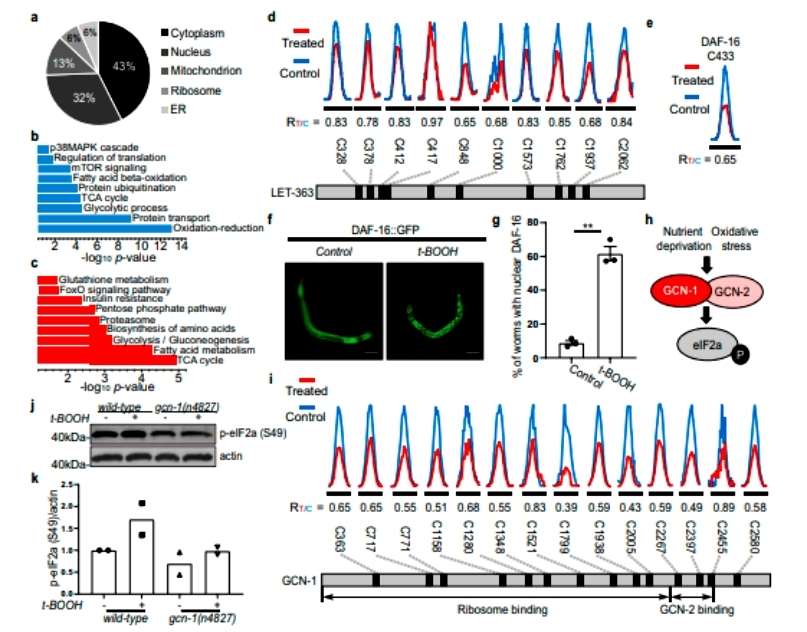 Figure 3
Figure 3
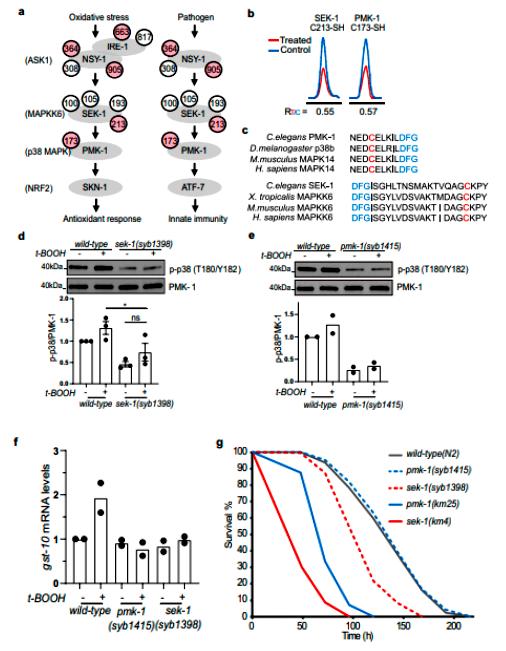 Figure 4
Figure 4
Conclusion
Cellular redox states and cysteine modifications are common during organismal aging, and cysteine redox profiles and redox state driver proteins may also be potential biomakers under physiological and pathological conditions.The authors' dataset of quantitative, site-centered mapping of intrinsic cysteine responsiveness and peroxide-sensitive cysteine redox forms greatly expands the the scope and biological role of cysteine oxidation in nematodes and provide a molecular basis for deciphering the complex redox signaling network in model organisms.
Reference
Our products and services are for research use only.