
- Home
- PTMs Proteomics
- Proteomics Analysis of γ-Carboxylation
γ-Carboxylation, a pivotal post-translational modification, plays a fundamental role in the biological activation of proteins involved in coagulation, bone metabolism, and vascular health. Governed by the vitamin K-dependent carboxylase enzyme, this process involves the addition of a carboxyl group to glutamic acid residues, transforming them into γ-carboxyglutamic acid (Gla). This modification confers unique functional properties to proteins, influencing their structure and interactions.
The mechanism of γ-carboxylation hinges on the availability of vitamin K, a fat-soluble essential nutrient. In the endoplasmic reticulum, the vitamin K-dependent carboxylase catalyzes the carboxylation of specific glutamic acid residues within target proteins. This modification is crucial for the biological activity of proteins like clotting factors, osteocalcin, and matrix Gla-protein.
γ-Carboxylation, driven by the interplay of vitamin K and specific carboxylase enzymes, orchestrates the functional diversity of proteins crucial for coagulation, bone health, and cardiovascular homeostasis. Analytical methods, ranging from mass spectrometry to western blotting, Creative Proteomics utilizes a variety of analytical methods to help researchers accurately interpret γ-carboxylation. As our understanding deepens, the applications of γ-carboxylation analysis extend beyond unraveling molecular mechanisms to informing diagnostic and therapeutic strategies in diverse physiological contexts.
Vitamin K-dependent carboxylation regulates Ca2+ flux and adaptation to metabolic stress in β cells
Journal: Cell Rep
Published: 2023
Background
The study investigates the role of vitamin K-dependent carboxylation in pancreatic β cells and its impact on insulin secretion. Recognizing the physiological inducer of ER stress, glucose, the research aims to understand how the vitamin K (VK) cycle, specifically involving Ggcx and Vkorc1 genes, is regulated in response to elevated glucose levels. Additionally, the study identifies a novel VK-dependent carboxylated protein, ER Gla Protein (ERGP), expressed in β cells.
Sample
The research uses a variety of samples to address its objectives. It employs wild-type C57BL/6J mouse islets, the rat β cell line INS-1 832/3, islets from 2-month-old Wistar rats infused with glucose, and non-diabetic human islets cultured in a medium containing high glucose. The study also relies on a mouse model with Ggcx deficiency in β cells (Ggcxff; Ins1-Cre) and utilizes CRISPR-Cas9 genome editing to create ASPH-knockout HEK293 cells, allowing for the examination of ERGP function.
Technical Approach
The study employs multiple techniques to explore the research questions. RNA-seq analysis, qPCR, and western blotting are used to assess gene expression and protein levels in response to glucose. Immunoprecipitation and mass spectrometry techniques identify ERGP and ASPH as potential γ-carboxylated proteins. The study utilizes immunofluorescence to confirm ERGP expression in islet endocrine cells. Functional experiments involve the manipulation of VK levels and the use of the VK antagonist warfarin. Techniques such as ratiometric Ca2+ imaging and FRET-based Ca2+ sensors are employed to study the impact of ERGP on Ca2+ flux and store-operated Ca2+ entry (SOCE).
Results
The author through Pancreatic islets exhibits high expression of GGCX and VKORC1 proteins, as identified through ProteomicsDB analysis. This aligns with mouse islet data indicating high expression of Ggcx and Vkorc1 genes. Fluorescence-activated cell sorting of β cells from mouse islets confirms Ggcx and Vkorc1 expression in β cells and other islet endocrine cells. Single-cell transcriptomics data and additional transcriptomics data corroborate these findings. GGCX and VKORC1 proteins are expressed at higher levels in purified β cells than in whole islets. The study demonstrates γ-carboxylation in pancreatic islets and specifically in β cells, indicating a functional vitamin K cycle. GGCX expression and γ-carboxylation are also observed in a rat insulinoma cell line and human islets. In vivo, experiments with Ggcx-inactivated mice confirm the presence of carboxylated proteins in islets and β cells. Human islets treated with vitamin K1 show increased protein γ-carboxylation, while warfarin, a VKORC1 inhibitor, reduces it. These findings suggest the presence of a functional vitamin K cycle in islets and highlight the importance of vitamin K in glucose metabolism and type 2 diabetes(Figure 1).
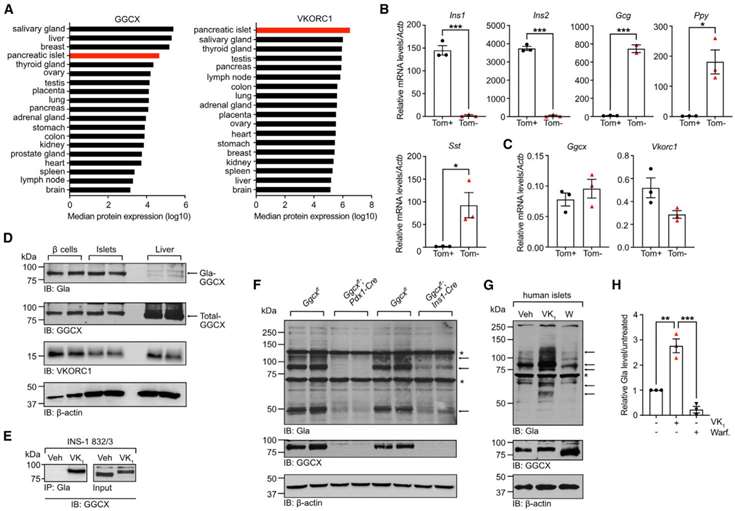 Figure 1
Figure 1
Mouse islet RNA-seq data indicate low expression of genes encoding known γ-carboxylated proteins, suggesting the presence of unknown Gla proteins in β cells. Immunoprecipitation from mouse liver extracts identifies aspartyl/asparaginyl b-hydroxylase (ASPH) as a putative intracellular Gla protein. ASPH has multiple isoforms, including a truncated version named ER Gla Protein (ERGP), expressed in pancreatic islets. ERGP is γ-carboxylated, as confirmed by immunoprecipitation and western blotting. ASPH and ERGP mRNAs are expressed in pancreatic islets, with ERGP being more abundant. Publicly available islet transcriptomics data show the expression of ASPH and ERGP in β cells. Antibodies against the negatively charged Glu-rich domain (GRD) of ASPH and ERGP confirm their γ-carboxylation. γ-carboxylation of ASPH and ERGP is demonstrated in WT mouse liver, adult control mouse islets, and stressed β cells. Islets from Vkorc1−/−; APOE-Vkorc1l1 mice, with lower VKORCs activity, show reduced γ-carboxylation. In Ggcxff; Ins1-Cre islets, ERGP is strongly γ-carboxylated in β cells. Immunofluorescence on WT mouse islets confirms ERGP expression in islet endocrine cells, including β cells. These findings establish ERGP and, to a lesser extent, ASPH as γ-carboxylated proteins expressed in mouse β cells(Figure 2).
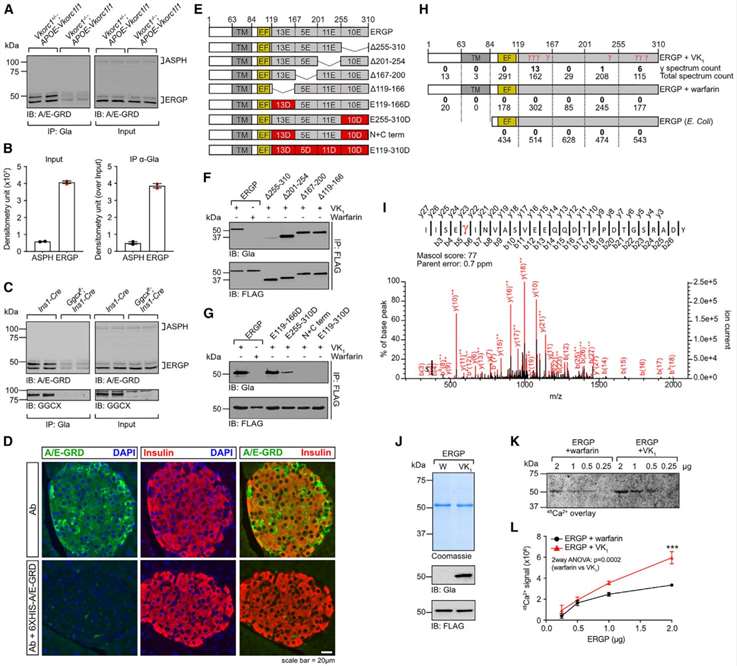 Figure 2
Figure 2
ERGP, a γ-carboxylated protein identified in mouse β cells, was investigated for its cellular function, as it had not been previously studied. In T cells, junctate, a related protein, was suggested to act as a Ca2+-sensing ER protein regulating store-operated calcium entry (SOCE). Given the potential role of SOCE in insulin secretion by β cells, the study aimed to explore whether ERGP and its γ-carboxylation could influence cellular Ca2+ flux and SOCE. To investigate this, CRISPR-Cas-9 genome editing was employed to knock out all ASPH isoforms in HEK293 cells, creating ASPH/HEK293 cells. The study utilized these cells to express STIM1-Myc and Orai1-hemagglutinin (HA) along with ERGP-33FLAG in the presence or absence of vitamin K1 (VK1).
Results indicated that γ-carboxylated ERGP in VK1-treated cells led to efficient γ-carboxylation, as confirmed by the detection of carboxylated GGCX. Ratiometric cytosolic Ca2+ measurements using Fluo-4 and Fura Red Ca2+ indicators revealed that cells expressing γ-carboxylated ERGP exhibited reduced ER Ca2+ release and SOCE. Additionally, there was a significant reduction in basal cytosolic Ca2+ levels in these cells, suggesting that γ-carboxylated ERGP plays a role in restraining cytosolic Ca2+ levels. These findings imply a potential regulatory function of carboxylated ERGP in the SOCE mechanism, highlighting its influence on cellular Ca2+ dynamics(Figure 3).
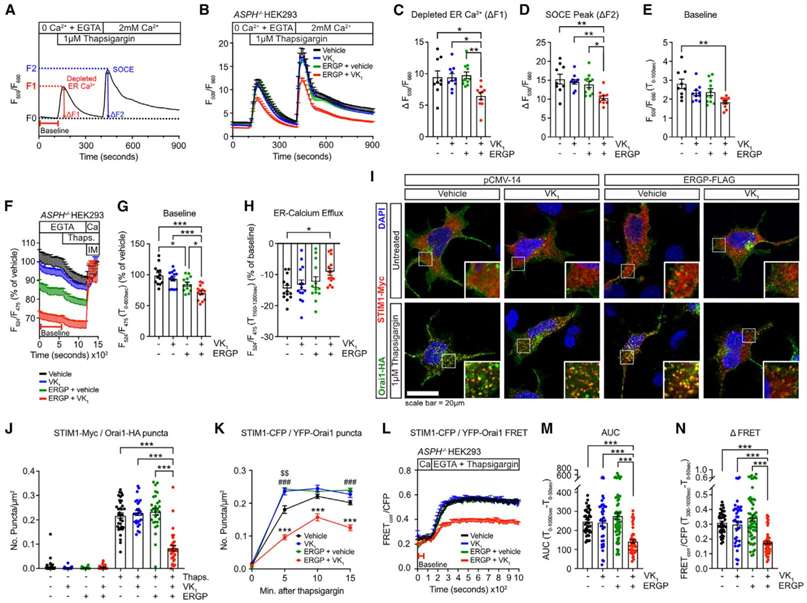 Figure 3
Figure 3
Using Ggcxff; Ins1-Cre islets as a genetic model of decarboxylated ERGP in β cells, the study aimed to investigate how the γ-carboxylation of ERGP affects Ca2+ homeostasis in these cells. Cytosolic Ca2+ flux was analyzed in partially dissociated islet cells from Ggcxff; Ins1-Cre and Ins1-Cre (control) mice through ratiometric live-cell imaging. SOCE was monitored with a protocol similar to that used for HEK293 cells, with the inclusion of diazoxide and verapamil in the buffered solution to prevent membrane depolarization and Ca2+ influx through voltage-gated Ca2+ channels.
Results showed that Ggcxff; Ins1-Cre β cells exhibited higher baseline cytosolic Ca2+ levels compared to control cells. Additionally, SOCE, expressed both as an absolute value and relative to baseline, was increased in Ggcxff; Ins1-Cre β cells. ER Ca2+ release, measured following treatment with a higher dose of thapsigargin, was significantly higher in these cells. The study also observed an elevated number of STIM1-containing puncta at steady state, indicating that γ-carboxylated ERGP restrains SOCE to maintain proper resting ER Ca2+ levels in β cells. These findings suggest a regulatory role of γ-carboxylated ERGP in controlling Ca2+ dynamics and homeostasis in β cells(Figure 4).
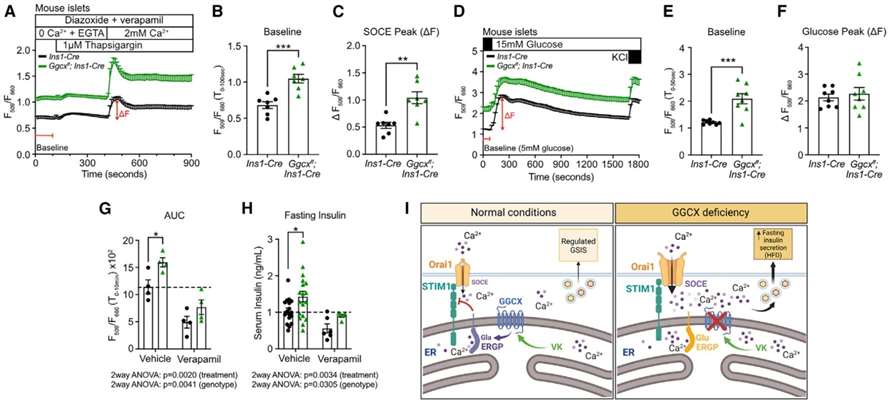 Figure 4
Figure 4
Conclusion
The results reveal that glucose positively regulates the VK cycle, inducing the expression of Ggcx and Vkorc1 in mouse islets and human islets. ERGP, identified as a novel γ-carboxylated protein, is expressed in β cells and is shown to be critical for maintaining Ca2+ homeostasis. Gamma-carboxylation of ERGP is found to restrain SOCE by modulating the interaction between STIM1 and Orai1, consequently impacting insulin secretion. In Ggcx-deficient β cells, increased basal cytosolic Ca2+ levels and elevated SOCE impair insulin secretion, a phenomenon reversed by the calcium channel blocker verapamil. Overall, the study establishes a link between vitamin K-dependent carboxylation, calcium flux regulation, and the adaptive capacity of β cells under metabolic stress.
Reference
Our products and services are for research use only.