
- Home
- PTMs Proteomics
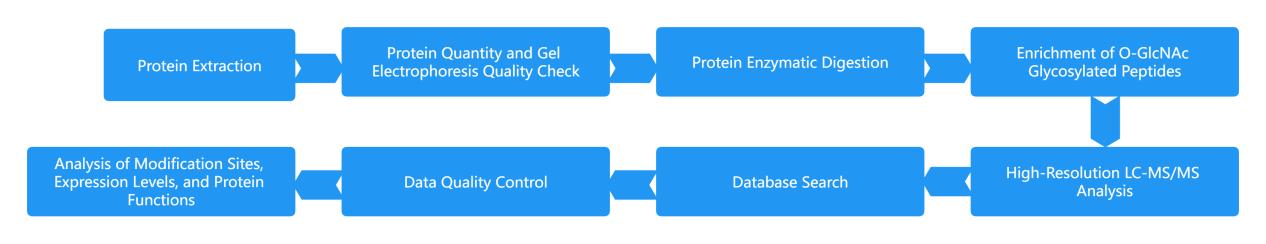
- Glycoproteomics Analysis
- O-GlcNAcylation Profiling Services
Protein glycosylation is one of the most important and prevalent post-translational modifications of proteins. This process, under the control of enzymes, involves the attachment of sugar chains to proteins and occurs in locations such as the endoplasmic reticulum and Golgi apparatus. Glycosyltransferases catalyze the transfer of sugars onto proteins, forming covalent glycosidic bonds with amino acid residues on the protein.
O-linked β-D-N-acetylglucosamine (O-GlcNAc) is the most common glycosylation modification. It occurs on serine (S) and threonine (T) residues of proteins through O-glycosidic bonds, where a single N-acetylglucosamine (GlcNAc) moiety is added. O-GlcNAcylation can modify thousands of proteins found in the cell nucleus, cytoplasm, cell membrane, and mitochondria. As a major "nutrient sensor" in cells, the O-GlcNAc pathway is sensitive to the metabolic state of the cell. O-GlcNAcylation regulates protein functions at multiple levels, including enzyme activity, energy metabolism, cell cycle, transcriptional activity, subcellular localization, protein-protein interactions, and degradation.
As a novel O-glycan modification, growing evidence indicates its crucial functions in stress responses, immune regulation, transcriptional control, and its significance in major diseases such as cancer and cardiovascular diseases. O-GlcNAcylation is increasingly recognized as a promising therapeutic target, making it a forefront topic in glycomics research. However, the intrinsic chemical characteristics of O-GlcNAc have posed challenges for large-scale analysis. Therefore, there is an urgent need to identify numerous O-GlcNAc proteins and their modification sites, and to explore their vital biological significance and regulatory functions. Consequently, O-GlcNAc modification glycomics research holds tremendous novelty, innovation, and application potential.
However, the challenge in analyzing modified protein components lies in the high abundance of modified peptide segments compared to non-modified ones, especially when the abundance of O-GlcNAc is much lower than that of phosphorylation modifications. Therefore, the selection of reliable and stable modification peptide enrichment antibodies is of utmost importance.
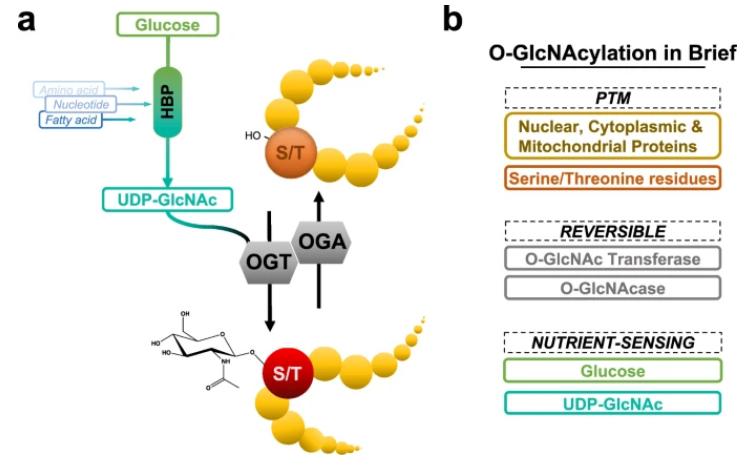 The O-GlcNAc cycling (Wulff-Fuentes, et al,. Sci Data 2021)
The O-GlcNAc cycling (Wulff-Fuentes, et al,. Sci Data 2021)
O-GlcNAcylation is the only intracellular glycosylation involved in signal transduction, occurring within cells (nucleus, cytoplasm, and mitochondria).
All eukaryotes (including animals, insects, and plants) exhibit protein O-GlcNAcylation. Some bacteria, fungi, and viruses also feature protein O-GlcNAcylation. O-GlcNAc exerts its influence in a species, tissue/cellular, protein, and site-specific manner.
Similar to a code, O-GlcNAcylation plays a crucial regulatory role in nearly all cellular processes.
Innovative Research Focus: Novel O-glycan modifications, "molecular switches" for target protein activity, with significant potential applications.
High-standard Modification Peptide Enrichment: Utilization of gold-standard antibody immunoprecipitation for the enrichment of modification peptide segments effectively addresses the labile nature of low-abundance O-GlcNAc, ensuring stability throughout the O-GlcNAc modification proteomics experiment.
High-Performance Mass Spectrometry Platform: Provides increased depth of identification, higher quantitative accuracy, and greater reliability in site-specific identification.
One-stop Service: Qualitative and quantitative characterization of O-GlcNAc sites and glycoforms, facilitating dual analysis of protein function regulation in conjunction with phosphorylation.
Cutting-edge 4D Technology Platform: The next-generation 4D mass spectrometry platform guarantees enhanced detection capabilities and precise localization of modification sites, leading to the discovery of more unknown O-GlcNAc sites.
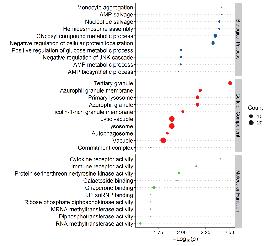 Gene Ontology Functional Analysis
Gene Ontology Functional Analysis
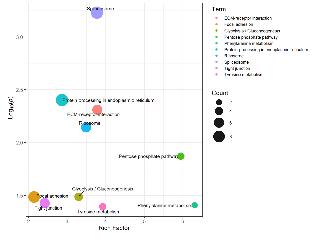 KEGG Pathway Analysis
KEGG Pathway Analysis
 Wikipathway Analysis
Wikipathway Analysis
 Reactome Analysis
Reactome Analysis
| Sample Type | Regular Submission Amount | Minimum Submission Amount |
|---|---|---|
| Conventional Animal Tissues (e.g., brain, heart, liver, spleen, lung, kidney, muscle, and skin, etc.) | 300 mg | 200 mg |
| Plant Tissues (e.g., leaves, flowers, etc.) | 800 mg | 500 mg |
| Cells | 8x10^7 | 6x10^7 |
| Pure Protein | 20 mg | 10 mg |
Case 1: Overall Approach: O-GlcNAc Modification Proteomics Analysis + Modification Validation + Functional Validation
Application: Investigation of Medical Disease Mechanisms
Title: Quantitative chemoproteomics reveals O-GlcNAcylation of cystathionine γ-lyase (CSE) represses trophoblast syncytialization
Journal: Cell Chemical Biology
Impact Factor: 9.039
Published: June 2021
Abstract:
It is known that defects in maternal O-GlcNAc glycosylation modification can lead to various adverse pregnancy events, such as preeclampsia and threatened miscarriage. The syncytialization of trophoblast cells is a critical period in early embryonic development, but the specific regulatory mechanism of O-GlcNAc modification in this process is not well understood.
Result
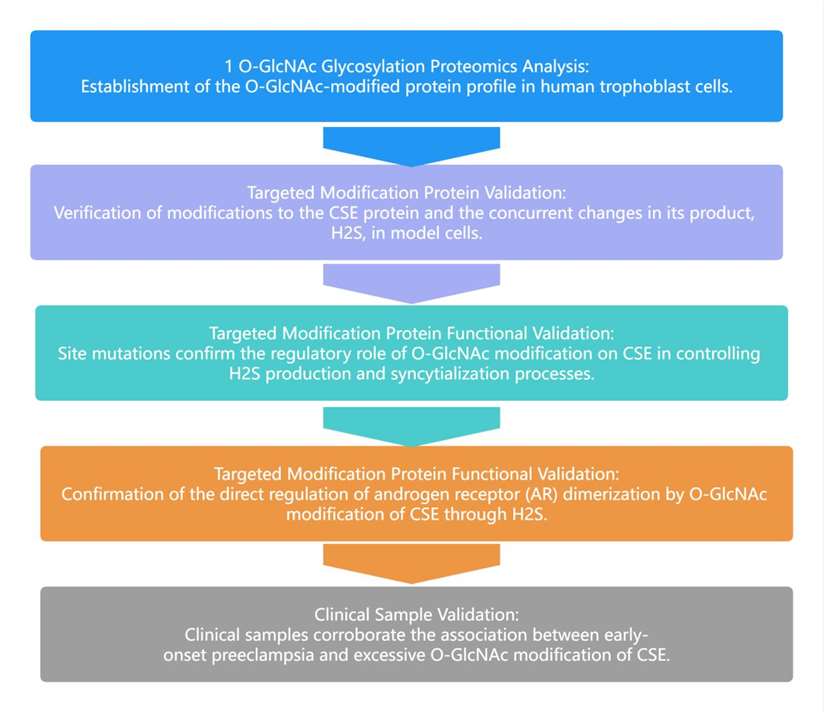
In this study, dynamic changes in modified proteins during trophoblast cell syncytialization were analyzed through O-GlcNAc protein modification proteomics. It was discovered that O-GlcNAc modification significantly upregulates cystathionine γ-lyase (CSE) during this process. Additionally, during trophoblast cell syncytialization, serine 138 of CSE is rapidly O-GlcNAc modified. This modification increases CSE activity and H2S production, further inhibiting the dimerization of the androgen receptor (AR) by H2S molecules, thus controlling the syncytialization of trophoblast cells. Abnormal elevation of CSE O-GlcNAcylation levels was also confirmed in clinical samples, closely related to inadequate syncytialization in patient placentas.
Research Approach
Case 2: O-GlcNAc Modification Proteomics + Phosphorylation Modification Proteomics + Bioinformatics Integration
Application: Agricultural Development Research
Title: The Protein Modifications of O-GlcNAcylation and Phosphorylation Mediate Vernalization Response for Flowering in Winter Wheat
Journal: Plant Physiology
Impact Factor: 8.4
Published: 2019
Abstract
It is known that O-GlcNAc glycosylation and phosphorylation play crucial roles in various biological processes individually, and O-GlcNAcylation can influence the phosphorylation status. However, their synergistic effects in response to external environmental signals in plants are poorly understood.
Result
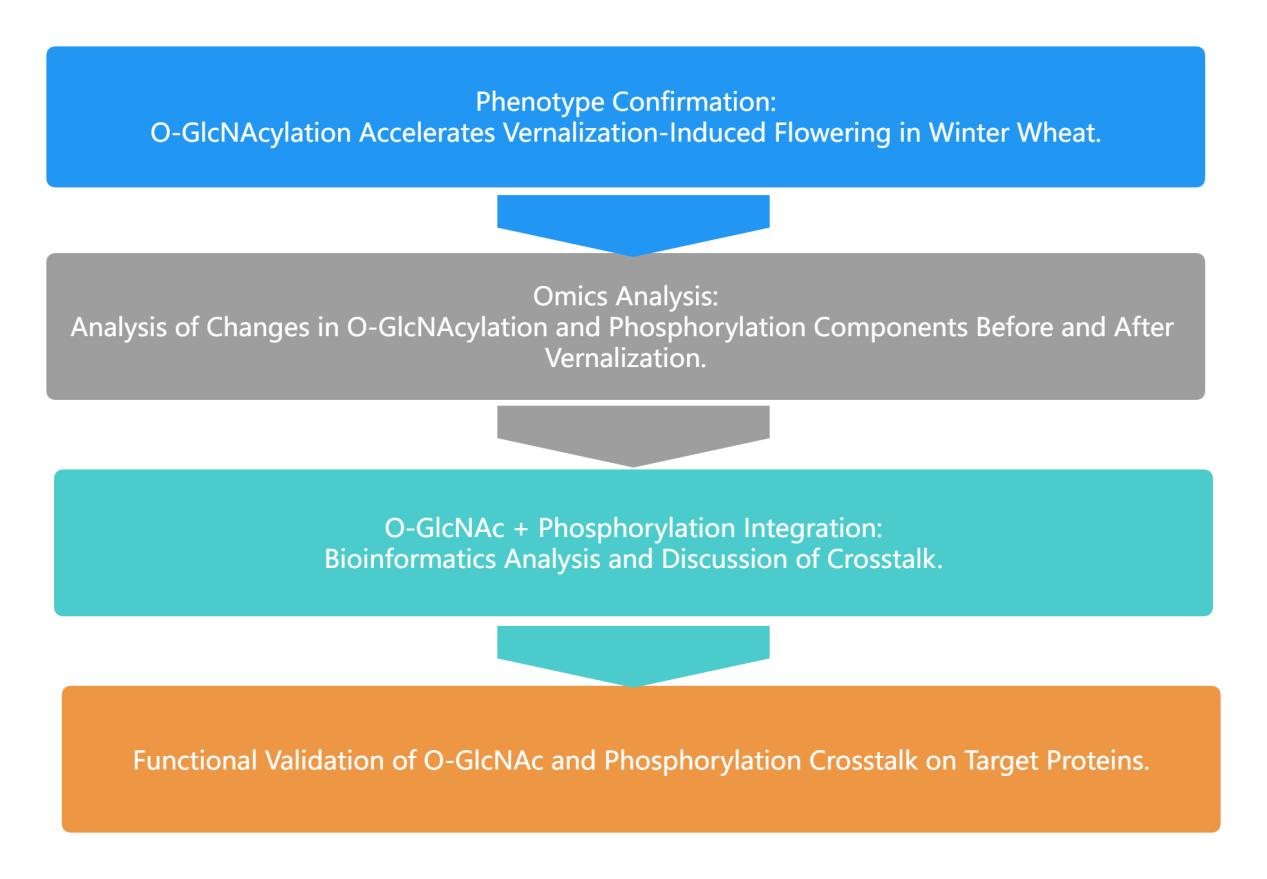
This study explores the role of O-GlcNAc glycosylation modification in the vernalization process and analyzes the dynamic distribution of O-GlcNAc glycosylation and phosphorylated proteins in the vernalization response. The results reveal that O-GlcNAc glycosylation modification affects the vernalization response, subsequently altering the expression of VRN genes, thus impacting flowering. O-GlcNAc glycomics and phosphorylation proteomics identified a variety of different O-GlcNAc and phosphorylated proteins, each participating in different signaling pathways. Furthermore, the study identified 31 vernalization-related proteins undergoing both phosphorylation and O-GlcNAc glycosylation modifications, with 3% of proteins having both modifications at the same site and the remaining 97% co-regulating protein functions. Co-modification occurred in TaGRP2 (O-GlcNAc at S87 and phosphorylation at S152), where site mutations affected TaGRP2's binding to TaVRN1 in vitro. In conclusion, dynamic networks of O-GlcNAc glycosylation and phosphorylation at key pathway nodes regulate wheat vernalization response and mediate flowering.
Research Approach
Our products and services are for research use only.