
- Home
- PTMs Proteomics
- Proteomics Analysis of Lysine Hydroxylation and Cross-Linking of Collagen
Collagen, a fundamental component of the extracellular matrix, plays a pivotal role in maintaining tissue integrity and function. Lysine hydroxylation and subsequent cross-linking are crucial post-translational modifications that profoundly influence collagen's structural and functional properties. Continued research in lysine hydroxylation and collagen cross-linking is poised to unveil novel insights into their roles in health and disease. Advanced analytical techniques and integrative approaches will likely contribute to a deeper understanding of the molecular intricacies governing collagen modifications, paving the way for innovative therapeutic strategies.
Collagen, primarily composed of three polypeptide chains, undergoes lysine hydroxylation, a process catalyzed by lysyl hydroxylases (LH). This enzymatic modification introduces hydroxyl groups to specific lysine residues, forming hydroxylysine. This modification is pivotal for stabilizing the collagen triple helix structure, imparting resilience to mechanical stress and conferring unique biophysical properties to various collagen types.
Lysine hydroxylation sets the stage for collagen cross-linking, a process that reinforces its structural integrity. Enzymes like lysyl oxidase (LOX) mediate the formation of covalent cross-links between adjacent collagen molecules, contributing to the tensile strength and stability of collagenous tissues. The cross-linking pattern varies among collagen types, influencing tissue-specific biomechanical properties.
Precise characterization of lysine hydroxylation and collagen cross-linking necessitates sophisticated analytical methods.
The impact of lysine hydroxylation and collagen cross-linking extends beyond structural considerations, influencing various physiological and pathological processes.

The dynamic interplay between lysine hydroxylation and collagen cross-linking orchestrates the intricate tapestry of extracellular matrix biology. Creative Proteomics, based on state-of-the-art technology platforms and specialized research services,and always stands at the forefront of unraveling the molecular signatures that underlie tissue function and dysfunction.
P3h3-null and Sc65-null Mice Phenocopy the Collagen Lysine Under-hydroxylation and Cross-linking Abnormality of Ehlers-Danlos Syndrome Type VIA
Journal: J Biol Chem
Published: 2017
Background
The collagen family of proteins has evolved into many different genes and gene translational products each with variably regulated post-translational modifications. Even within a single genetic type of collagen, the post-translational quality of the protein is known to be regulated with a high degree of tissue specificity. The functional significance of this post-translational variability is still not fully understood. Then, it highlights the significance of prolyl 3-hydroxylation, a relatively rare collagen modification, and its connection to recessive forms of osteogenesis imperfecta. Mutations in genes such as P3H1, CRTAP, and CypB are linked to collagen-related disorders.
The study explores P3H2 mutations causing high myopia and investigates the substrate specificity and function of P3H3 and Sc65 within the collagen family. The research involves screening tissues from Sc65- and P3h3-null mice using mass spectrometry to identify collagen post-translational effects. The results indicate severe under-hydroxylation of lysines, particularly at cross-linking sites in type I collagen across various tissues. The findings suggest a complex interplay between proteins involved in collagen prolyl 3-hydroxylation and lysyl hydroxylation in the endoplasmic reticulum, shedding light on the evolution and regulation of collagen-related processes.
Results
The authors describe the generation of Sc65 and P3H3 knockout mice. For Sc65, two models were created: one using gene trap insertional mutagenesis and the other through the introduction of loxP sites flanking the Leprel4 gene. Both Sc65-null mouse models were viable with no apparent skeletal defects, exhibiting normal growth and behavior. For P3H3, inactivation was achieved using homologous recombination in ES cells through BAC technology. The resulting P3h3-null mice were viable and displayed no observable abnormalities. Immunoblot analysis confirmed the absence of P3H3 protein expression in P3h3−/− mice. Histological examination of P3h3−/− skin sections indicated collagen fabric fragility similar to Sc65−/− skin sections. Mechanical testing revealed decreased structural integrity in the skin of both null strains. The study utilized tissues from heterozygous P3h3+/− mice as a control, demonstrating similarity to wild-type (Sc65+/+) tissues in SDS-PAGE and mass spectral analysis (Figure 1).
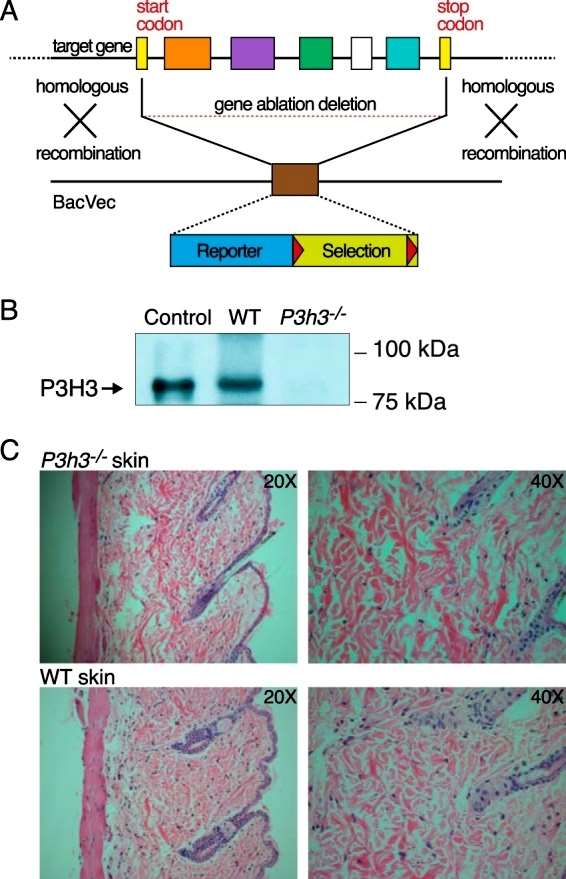 Figure 1
Figure 1
The study analyzed acid-extracted collagens from skin, bone, and tendon using SDS-PAGE to investigate potential tissue-specific differences in collagen cross-linking profiles. Comparisons between Sc65−/− and P3h3−/− mice and their respective wild-type (WT) littermates revealed reproducible and similar differences in collagen chain patterns, particularly in the skin. Notably, a previously identified chain trimer, γ112, was consistently more prominent in collagen extracts from null mice compared to the α and β bands. The β12 component in null strains showed a noticeable depletion in proportion to the increase in γ112. This shift from β12 to γ112 suggested an impact on intermolecular cross-linking, as observed in fibromodulin null mouse tendons. The intensity shift was most pronounced in skin, less evident in tendon, and absent in bone collagen extracts (Figure 2).
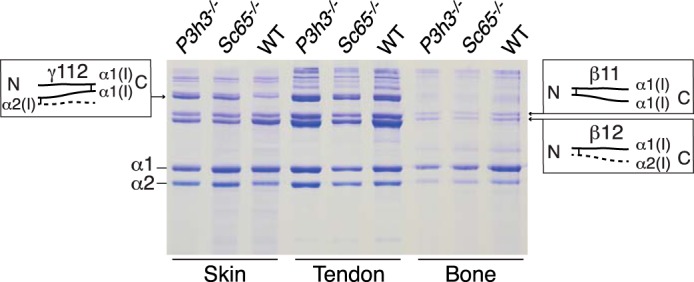 Figure 2
Figure 2
The study utilized tandem mass spectrometry to investigate lysine hydroxylation at the cross-linking site, specifically residue Lys-87, in type I collagen α1(I) chains extracted from various tissues (bone, skin, tendon, aorta, and cornea) from Sc65−/− and P3h3−/− mice. A consistent pattern of reduced hydroxylation was observed in both null mice, with a more pronounced effect in Sc65−/− tissues. In skin, the post-translational shift at helical domain cross-linking residue Lys-87 was more significant compared to bone. Type I collagen from soft tissues (skin, tendon, and aorta) exhibited almost complete loss of post-translational modification at Lys-87 in both Sc65−/− and P3h3−/− mice. Additionally, all helical cross-linking sites appeared to be under-hydroxylated in the P3h3−/− mouse. The study highlighted the specific impact on hydroxylation at Lys-930 in skin, bone, and tendon from both P3h3−/− and Sc65−/− mice, with tendon from wild-type mice showing unique characteristics (Figure 3).
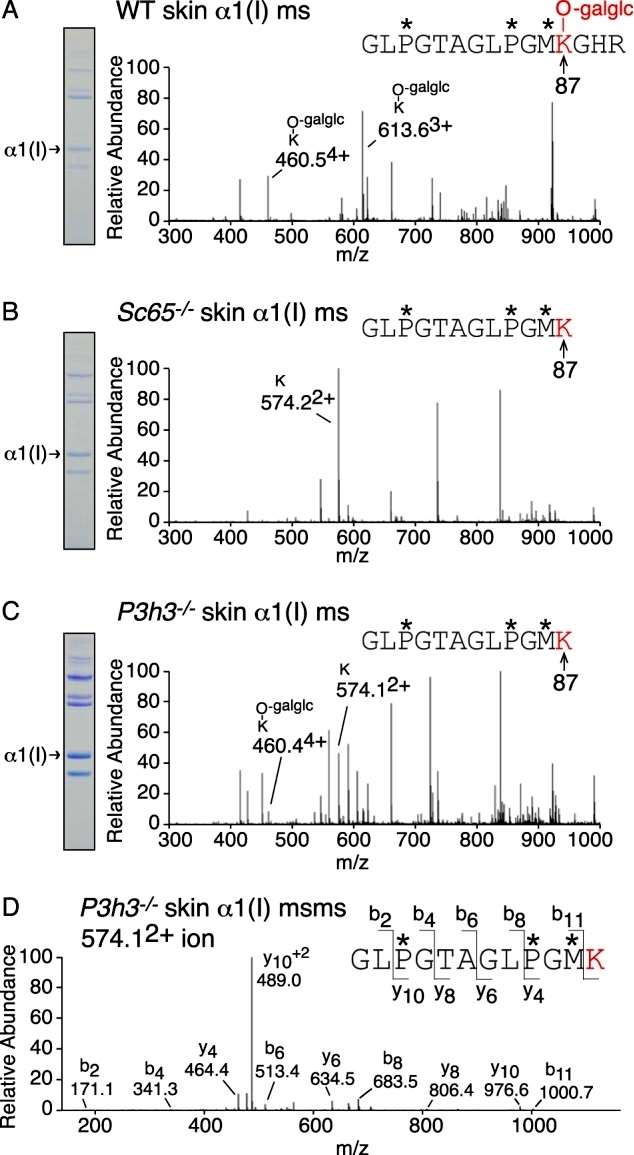 Figure 3
Figure 3
The study employed mass spectrometry analysis of bacterial collagenase digests of borohydride-reduced skin to examine the pool of aldimine divalent cross-links in whole tissue collagen. This approach aimed to track structural intermediates in the lysine aldehyde cross-linking pathway. In wild-type (WT) skin, the LC-MS profile revealed a fully glycosylated reduced aldimine cross-linked structure between α1(I) Lys-87 and an α1(I) C-telopeptide lysine, consistent with the prevalence of glcgal-Hyl at Lys-87 in the linear tryptic peptide from the α1(I) chain in WT skin. In P3h3−/− mouse skin, equal amounts of the fully glycosylated and a non-glycosylated reduced aldimine cross-link structure were recovered, indicating a chemical and steric preference for glcgal-Hyl at Lys-87. The total ion current suggested significantly fewer aldimine cross-links at this site in mutant skin compared to WT skin. Additionally, the cross-link between α1(I) Lys-930 and the α1(I)N-telopeptide, fully hydroxylated in WT skin, was identified as only the non-hydroxylated aldimine cross-link in P3h3−/− mouse skin (Figure 4).
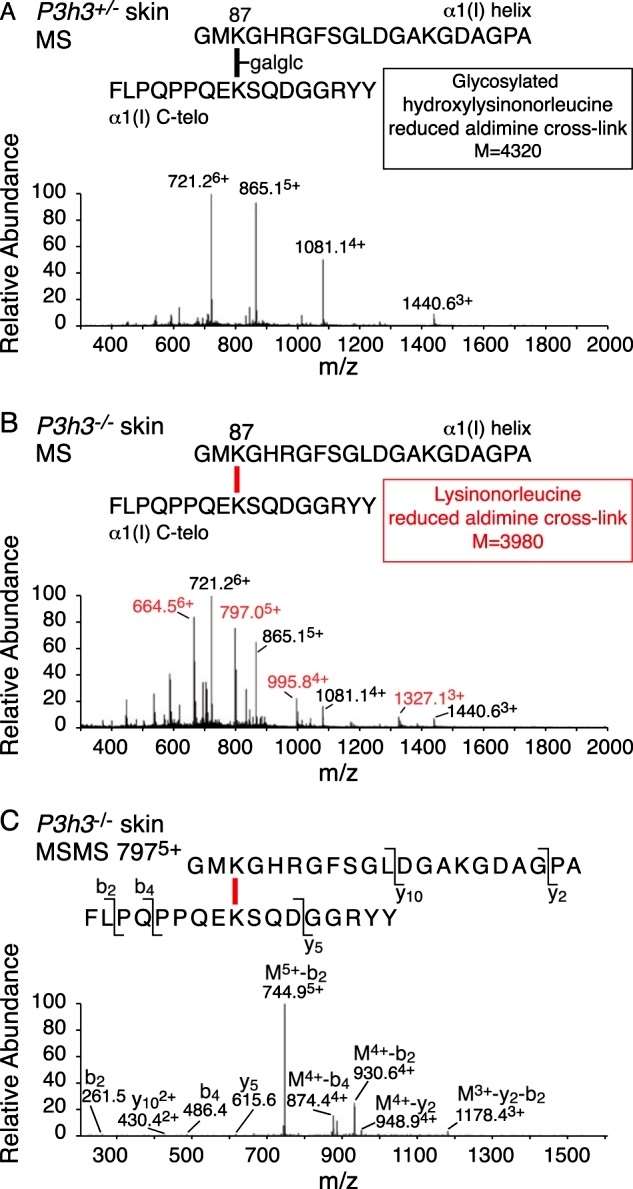 Figure 4
Figure 4
Conclusion
The article employs tandem mass spectrometry on tissues from targeted mutant mouse models to investigate collagen substrate specificities within the prolyl 3-hydroxylase (P3H) gene family. Previous findings established that P3h1 and P3h2 have distinct roles in 3-hydroxylating proline sites in collagen types I and multiple collagen types (I, II, IV, and V), respectively. Screening P3h3 and Sc65 knock-out mice reveals a common effect of lysine under-hydroxylation at cross-linking sites in various tissues. While prolyl 3-hydroxylation remains unaffected, collagen type I from mutant skin displays abnormal chain patterns with altered cross-link chemistry. The study indicates a reversal in the ratio of mature cross-links in bone, comparable to observations in certain human and mouse tissues, suggesting potential links between P3H3/SC65 mutations and yet-to-be-defined variants of Ehlers-Danlos Syndrome (EDS).
Reference
Our products and services are for research use only.