
- Home
- PTMs Proteomics
- Proteomics Analysis of Selenylation
A vital biochemical process called selenium incorporation into biological molecules is called seleniumylation, and it is essential for many physiological processes. Selenylation is tightly regulated within cells. Investigating the regulatory mechanisms governing selenoprotein synthesis provides insights into cellular adaptation to varying selenium levels and helps understand the consequences of dysregulation. Ongoing advancements in analytical technologies, such as the integration of multi-omics approaches, contribute to a more comprehensive understanding of selenylation dynamics. This enables researchers to explore intricate interactions between selenoproteins and other cellular components.
Precise analysis of selenylation is paramount for unraveling its mechanisms and implications. Several sophisticated analytical techniques are employed in elucidating selenium-containing compounds:
Understanding selenylation has far-reaching implications across various scientific domains, including as follows.
The study of selenylation holds paramount significance across various scientific disciplines, ranging from molecular biology to human health and environmental science. Understanding the intricate processes of selenylation provides critical insights into the role of selenium in biological systems.
Selenylation stands as a captivating field of study, with its implications extending from molecular biology to human health and environmental science. Creative Proteomics employs cutting-edge analytical methods to help researchers unravel the intricacies of selenylation, opening avenues for innovative applications and furthering our understanding of this essential biochemical process. The insights gained from selenylation research have the potential to reshape our understanding of selenium biology and influence diverse fields, ultimately contributing to improved well-being and a deeper comprehension of life processes.
Facultative protein selenation regulates redox sensitivity, adipose tissue thermogenesis, and obesity
Journal: PNAS
Published: 2020
Background
The thermogenic process in brown adipose tissue (BAT) has attracted the attention of scientists as it can combat obesity and diabetes. Recent studies of physiological reactive oxygen species (ROS) levels in BAT have revealed that redox signaling plays an important role in thermogenesis in vivo and that this regulation involves oxidative modification of sensitive Cys residues in target proteins. Selenocysteine (Sec), an analog of Cys, is more reactive than Cys but is thought to be typically found only in very small amounts in selenoproteins. To investigate the possible role of Sec in the regulation of adipose tissue thermogenesis by ROS, the authors set out to study the status of selenoproteins and Sec during BAT thermogenesis.
Results
First, the authors developed a mass spectrometry method to detect the presence of Sec in proteins.Sec, similar to Cys, reacts with alkylating reagents for thiols such as iodoacetamide (IAM) and N-ethylmaleimide (NEM), and thus Sec-containing peptides can theoretically be identified in mass spectrometry by the difference in mass corresponding to the substitution of Cys for Sec plus the mass modified by the corresponding alkylating reagent. segments. In addition to this, the isotopic distribution of selenium is very unique in nature, and this isotopic distribution feature can also be identified using mass spectrometry. The authors then used the mass difference and isotopic distribution as a basis for determining the presence of Sec. The authors first verified the feasibility of the method on the mouse selenoprotein Gpx1 and found that the site of Sec could be easily identified; then the authors used the method for mass spectrometry analysis of the UCP1 protein in BAT, as the authors had observed that part of the Cys of UCP1 had been replaced by Sec in a previous study. After NEM derivatization, mass spectrometry identified six of the seven Cys of UCP1, and Cys253 was identified as Sec because it exhibited a Cys-to-Sec mass difference as well as an isotopic distribution of selenium signatures.The experimental results showed that selenylation occurred in approximately 4-5% of the total UCP1 protein in BAT from wild-type mice, and that Sec253 was more susceptible to oxidation compared to Cys to the form of selenite (Figure 1).
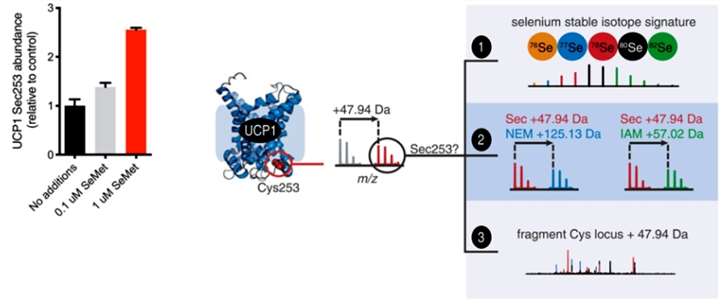 Figure 1
Figure 1
Next, the authors investigated how Sec is inserted into non-selenoproteins like UCP1. After treating BAT with 75Se-labeled sodium selenite, the authors did not observe a radioactive signal in the mass region corresponding to UCP1, but the Sec signature was still detectable by mass spectrometry, suggesting that Sec incorporation into UCP1 is a parthenogenetic process that is independent of Sec translation in normal selenoproteins. When the authors supplemented the cells with selenomethionine instead of inorganic selenium, the proportion of Sec253 forms of UCP1 increased significantly.
As the expression of a number of known selenoproteins can be readily regulated by dietary selenium supplementation, the authors naturally wondered whether the extent of protein selenization in BAT could also be regulated by controlling the diet. For the specialized selenoproteins, the authors found that their expression was indeed dependent on dietary selenium availability; for other possible parthenogenetic selenoproteins in the BAT proteome, the authors also found multiple parthenogenetic Sec insertion sites, and the proportion of their occurrence was also dependent on selenium intake. Repeated experiments showed that these sites are not random but highly specific, suggesting that selenium may be present in proteins more widely than previously recognized. Notably, some sites appeared on key residues of core metabolic proteins and may be potential redox regulatory sites. In addition to this, the authors identified a number of specific selenomethionine insertion sites. The authors attempted to characterize these parthenogenetic selenomethionine insertion sites at the genomic level, but found that almost all proteins did not contain the SECIS elements that characterize classical selenoproteins, and no common motifs were found in the structures of the different genes (Figure 2).
 Figure 2
Figure 2
Conclusion
The authors investigated the effects of protein parthenogenetic selenization on physiological metabolic processes such as adipose tissue thermogenesis. Adipose tissue thermogenesis was induced in mice fed diets supplemented with different concentrations of selenium for a period of time, and it was found that oxygen consumption and CO2 release were increased in the group with higher levels of parthenogenetic protein selenidation, suggesting that parthenogenetic protein selenidation promotes thermogenesis. After combining high selenium intake with a high-fat diet, the authors also found that increased levels of parthenogenetic protein selenization facilitated the avoidance of obesity. In conclusion, the authors found that selenium regulates important physiological processes such as energy expenditure and adipose tissue thermogenesis through parthenogenetic selenoproteins.
Reference
Our products and services are for research use only.