
- Home
- PTMs Proteomics
- Proteomics Analysis of Neddylation
Neddylation is a novel post-translational modification of proteins that has been discovered in recent years and can regulate a wide range of biological processes, such as cell cycle, signaling and immune recognition. The ubiquitin-like protein NEDD8 binds to target proteins through a process similar to ubiquitination. NEDD8 and ubiquitin share 59% sequence identity. Neddylation is essential for several physiological functions, such as signal transmission, DNA repair, and cell cycle progression. Its role in diseases—especially cancer and neurological disorders, where abnormal neddylation patterns contribute to pathogenesis—underlines its significance.
Understanding the complexities of neddylation necessitates sophisticated analytical methods. Our researchers employ a range of techniques to dissect neddylation events at the molecular level as follows.
The implications of neddylation extend beyond fundamental cellular processes, finding applications in various realms of biomedical research:
Neddylation stands as a testament to the complexity of cellular regulation. Creative Proteomics employs cutting-edge analytical methods to help researchers provide researchers with the tools to dissect neddylation events with unprecedented precision. From cancer therapeutics to antiviral strategies, the applications of neddylation in biomedical research underscore its significance in health and disease. As we delve further into the regulatory mechanisms and crosstalk associated with neddylation, the potential for therapeutic innovations beckons, promising a deeper understanding of cellular processes that shape life at its molecular core.
Neddylation of phosphoenolpyruvate carboxykinase 1 controls glucose metabolism
Journal: Cell Metab
Published: 2023
Background
Glucose is the main source of cellular energy, and metabolic reprogramming occurs in the liver during fasting, using the gluconeogenesis pathway to mobilize non-carbohydrate precursors to synthesize glucose from scratch in order to maintain glucose homeostasis and to meet the body's energy requirements. Although some studies imply that neddylation plays a key role in hepatic energy metabolism, insulin signaling, nonalcoholic fatty liver development, and hepatic fibrosis, how the neddylation signaling pathway finely regulates metabolic adaptation and glucose metabolism remains to be elucidated.
Results
The authors first investigated whether nutritional adaptation affects neddylation levels and found that starvation treatment upregulated hepatic neddylation in mice, whereas refeeding decreased neddylation, a change mediated by a "leptin-receptor"-dependent mechanism. Inhibition of hepatic neddylation by targeting NAE1 or NEDD8 resulted in decreased gluconeogenesis in mice, leading to lower blood glucose levels. In addition, inhibition of hepatic neddylation after fasting did not alter gluconeogenic enzymes or acetyl coenzyme A levels, and the amount of amino acids important substrates for gluconeogenesis in the liver did not change in response to reduced neddylation. Given that inhibition of hepatic neddylation decreases gluconeogenic activity, the authors further explored whether neddylation is involved in the regulation of glucose antagonistic responses and found that neddylation mediates the actions of glucose-antagonistic hormones (epinephrine, and glucocorticoids) and is required for hepatic gluconeogenesis, and that activation in liver of Activation of neddylation in the liver promotes gluconeogenesis and inhibits the hypoglycemic effect of insulin.
Failure of insulin inhibition of hepatic gluconeogenesis in diabetic patients results in fasting and postprandial hyperglycemia. The authors examined hepatic neddylation levels in obese patients with type 2 diabetes mellitus and in normoglycemic obese adults and found that the degree of hepatic neddylation was elevated in obese patients with type 2 diabetes mellitus without gender preference. To elucidate how neddylation affects glucose metabolism, the authors next utilized neddylation proteomics to characterize the mapping of changes in hepatic protein neddylation in the gluconeogenesis-induced state. The results showed that the degree of neddylation modification of 61 proteins was altered after nutrient stress and was significantly enriched in the glycoheterotrophic pathway, which was represented by the rate-limiting enzyme of the glycoheterotrophic response, PCK1. Specifically, PCK1 had increased binding activity to NAE1 upon nutrient stress, which in turn was highly neddylated, and the modification was required for PCK1-mediated glycoheterotrophic response activity. The authors next identified the neddylation site of PCK1 using immunoprecipitation and tandem mass spectrometry, and found that the K278, K342, and K387 lysine residues located on the surface of PCK1 are capable of being modified by neddylation under starvation, and that all three residues are essential for PCK1 to regulate the gluconeogenic response and mouse glucose homeostasis under both in vivo and in vitro conditions (Figure 1) .
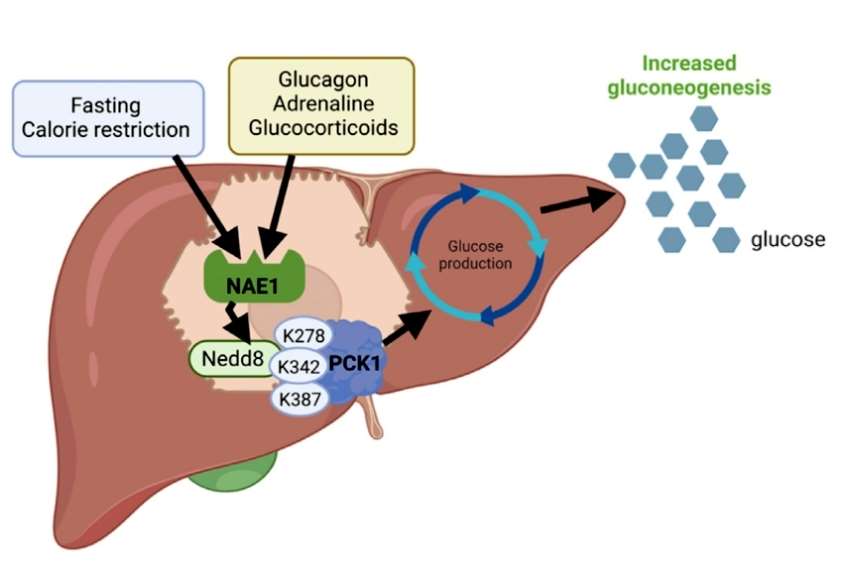 Figure 1
Figure 1
Conclusion
In summary, the present study found that the level of neddylation modifications is up-regulated in the livers of mice subjected to nutritional stress (fasting or caloric restriction) and type 2 diabetes mellitus and induces neddylation modification of residues K278, K342, and K278 of the gluconeogenesis reaction-limiting enzyme PCK1, which in turn repositions the two rings around the catalytic center into an open structure, allowing easier access of the substrate to the catalytic center, and promotes Glucose synthesis. The present study reveals that the neddylation modification of PCK1, the gluconeogenic rate-limiting enzyme, precisely regulates hepatic glucose metabolism by integrating overall nutrient supply and metabolic homeostasis.
Reference
Our products and services are for research use only.