
- Home
- PTMs Proteomics
- Proteomics Analysis of Butyrylation
Post-translational modifications (PTMs) are pivotal mechanisms influencing protein structure and function. Among these, butyrylation, the addition of butyryl groups to proteins, emerges as a dynamic process with far-reaching implications. Butyrylation involves the transfer of butyrate molecules to lysine residues, thereby modulating protein activity and cellular processes.
Butyrylation stands as a multifaceted post-translational modification with far-reaching implications in cellular processes. Analytical methods, ranging from mass spectrometry to immunological approaches, unravel the complexity of the butyrylome. Applications in epigenetic regulation and metabolic signaling underscore the significance of butyrylation in orchestrating cellular responses. Creative Proteomics relies on an advanced technology platform and a professional research team, we are aim to help researches recognize its dynamic nature and interconnectedness with other PTMs is paramount for fully understanding the functional impact of butyrylation in cellular physiology. As technology advances, the exploration of butyrylation promises to unveil novel dimensions in the intricate landscape of post-translational modifications.
Lysine butyrylation of HSP90 regulated by KAT8 and HDAC11 confers chemoresistance
Journal: Cell Discovery
Published: 2023
Background
Post-translational modification of proteins plays a very important role in living organisms, which makes the structure of proteins more complex and functional. Among them, lysine acylation modification refers to the enzyme-catalyzed transfer of acyl-coenzyme A (Acyl-CoA) donors to the lysine side chain of proteins, thereby altering the properties and functions of the proteins, a process that involves a variety of physiopathological processes. Lysine butyrylation modification (Kbu) is an important lysine acylation modification; however, its role in tumors is unclear and deserves in-depth study.
Results
First the team tested ESCC chemosensitive and resistant cell lines using 11 modification pan-antibodies and found only significant changes in butyrylation modification levels. The researchers then performed systematic proteomics and butyrylation modification histology analyses on the parental cells, KYSE150, as well as the 5-Fu-resistant cells, KYSE150-FR. In total, the study identified 123 modification sites of 105 proteins with significant differences.
The research team next performed more in-depth bioconfidence mining of the histological data to identify key proteins and key modification sites associated with chemoresistance. Protein interaction network analysis and GO enrichment analysis indicated that HSP90 may play an important role in chemoresistance. Examination of clinical samples revealed higher expression of HSP90 in relapse samples, and the butyrylated modification of HSP90 was significantly upregulated in 5-Fu-resistant samples. The above results suggest that the butyrylation modification of HSP90 may play a key role in ESCC chemoresistance (Figure 1).
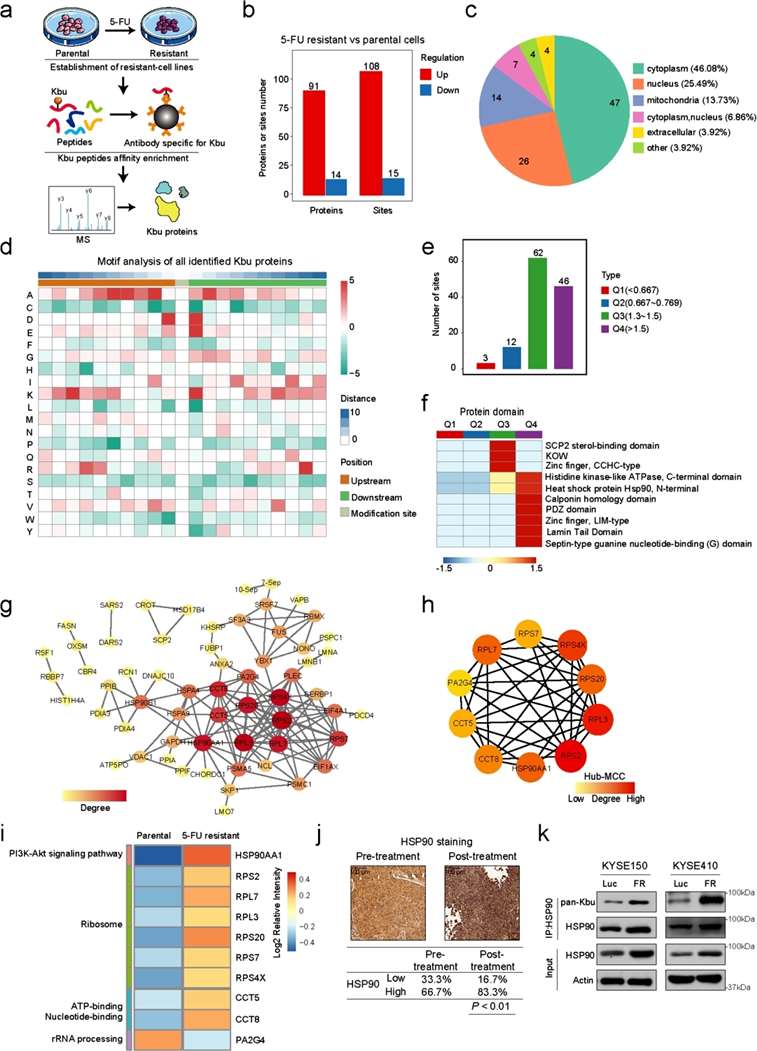 Figure 1
Figure 1
Mass spectrometry analysis revealed the presence of four butyrylation modification sites in the HSP90 protein, and only when the K754 site was mutated to arginine R (mimicking that no butyrylation modification would occur) did it significantly inhibit tumor drug resistance. In addition, HSP90 K754 is highly conserved in evolution from African clawed toad to human, suggesting the functional importance of this locus. Cloning experiments as well as tumor xenograft experiments further demonstrated that HSP90 overexpression increased 5-FU resistance in ESCC cells, whereas mutation of K754 to R754 resulted in a decrease in 5-FU resistance in tumor cells. To explore the mechanism behind this phenotype, the researchers used point mutation experiments and found that HSP90 K754R significantly destabilized the HSP90 protein, prompting its degradation by ubiquitination. This suggests the importance of the K754 butyrylation modification for maintaining the stability of the HSP90 protein. Thus, the butyrylation modification of HSP90 K754 plays a key role in ESCC chemoresistance and may function by maintaining the stability of HSP90 (Figure 2).
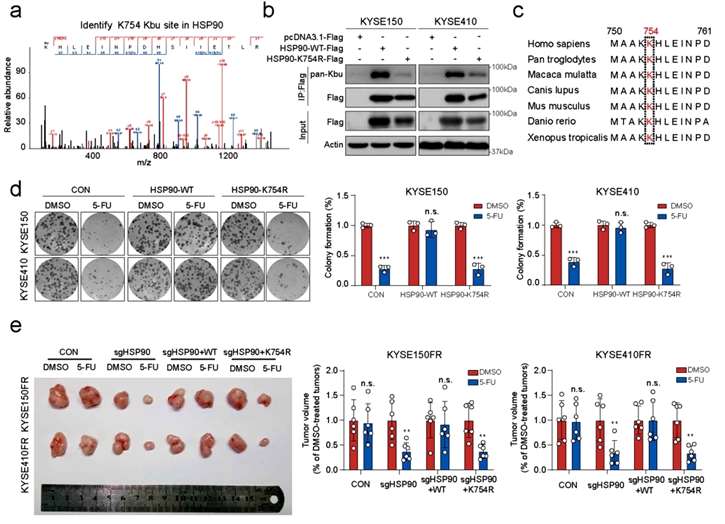
Biochemical experiments revealed that the expression of KAT8 differed between resistant and parental cells, but not that of HDAC11. Therefore, the researchers hypothesized that there may be other proteins that co-regulate the butyrylation modification and protein stability (ubiquitylation level) of HSP90 with HDAC11, and suggested that there may be a potential correlation between post-translational modifications of different proteins.
In order to identify the binding proteins of HSP90, the researchers performed immunoprecipitation followed by mass spectrometry (IP-MS) of HSP90 from drug-resistant ESCC cell lines, and analyzed the obtained data in conjunction with butyryl modification histology data, and identified a candidate protein, SDCBP, which was verified to bind to HSP90 by the IP assay. And it was found that HSP90 did not affect the stability of SDCBP, instead, SDCBP affected the stability of HSP90. These results suggest that SDCBP is an important factor leading to HSP90 butyrylation and protein degradation (ubiquitination level) (Figure 3).
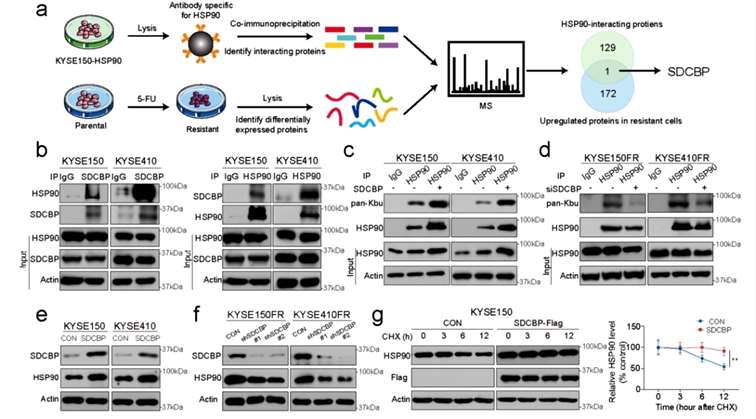 Figure 3
Figure 3
Although SDCBP competes with HDAC11 for binding HSP90, its role in chemoresistance is unclear. In vitro and in vivo experiments showed that overexpression of SDCBP enhanced cell resistance to 5-FU, and knockdown of SDCBP affected cell proliferation and cell viability. Mechanistic studies revealed that overexpression of SDCBP activated the AKT signaling pathway and up-regulated the expression of TS proteins, which caused chemoresistance in ESCC. knockdown of HSP90 reversed these features, suggesting that HSP90 is regulated by SDCBP, which plays a key role in the resistance to 5-FU through the AKT signaling pathway and TS proteins. The study further confirmed the important role of SDCBP in chemotherapy resistance in clinical samples by IHC staining and analysis of survival curves. In addition, the study yielded a lead compound (V020-9974) targeting HSP90 butyrylation through a high-throughput compound drug screening system. This compound reduced the binding of HSP90 to SDCBP, thereby inhibiting the level of butyrylation modification of HSP90 and enhancing the sensitivity of cells to 5-FU. These results indicate that inhibition of HSP90 butyrylation modification is a potential new strategy against 5-Fu resistance, suggesting that novel acylation modifications are important tools to fuel the field of precision medicine in oncology (Figure 4).
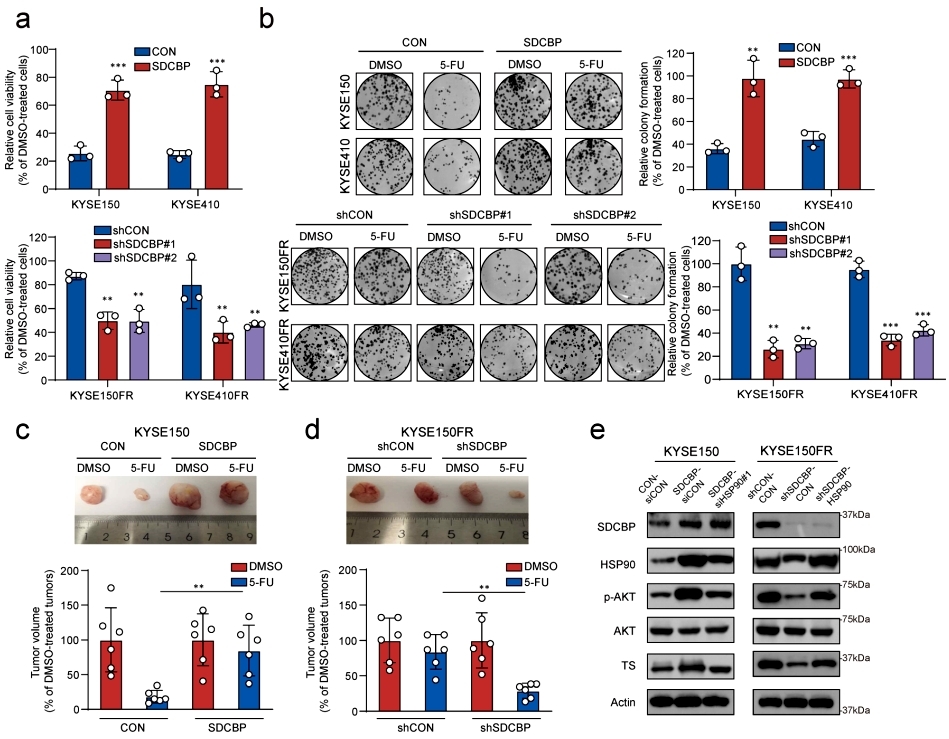 Figure 4
Figure 4
Conclusion
This study is the first systematic analysis of butyrylation modification in chemoresistant and chemosensitive ESCC cells, and identified a key factor regulating tumor 5-Fu resistance: butyrylation modification at the K754 position of HSP90. The study also identified SDCBP as a key factor driving the butyrylation modification of HSP90, which led to the up-regulation of the level of HSP90 butyrylation modification through competitive binding of HSP90 with HDAC11, further maintaining the stability of HSP90. A high-throughput compound screening system was also used to obtain the small molecule compound V020-9974, which inhibits the binding of SDCBP-HSP90, thereby decreasing the level of HSP90 butyrylation, leading to its ubiquitination and degradation, and inducing cellular chemosensitivity. This study not only opens a new chapter of butyrylation modification in cancer research, but also provides an important case study and a valuable resource for the study of butyrylation modification in chemosensitivity/resistance.
Reference
Our products and services are for research use only.