
- Home
- PTMs Proteomics
- Proteomics Analysis of Pyroglutamate Formation
Pyroglutamate, also known as pyroglutamic acid or 5-oxoproline, is a cyclic derivative of glutamic acid. Pyroglutamate is characterized by a cyclic amide structure, arising from the intramolecular reaction between the α-amino group and the carboxyl group of the same glutamic acid residue. This cyclization results in the loss of water and the formation of a stable five-membered ring structure. This intriguing molecule has garnered significant attention due to its involvement in various biological processes and its association with diverse physiological and pathological conditions. Understanding the formation of pyroglutamate is crucial for unraveling its role in cellular homeostasis and disease pathology.
Accurate detection and quantification of pyroglutamate are pivotal for comprehending its physiological relevance. Various analytical methods have been employed to discern pyroglutamate formation in biological samples.
Understanding pyroglutamate formation has far-reaching implications in various biological and clinical contexts.
Unraveling the intricacies of pyroglutamate formation is crucial for advancing our understanding of its biological significance. Analytical methods, ranging from mass spectrometry to ELISA, play pivotal roles in deciphering pyroglutamate's involvement in health and disease. The applications span from neurodegenerative diseases to biopharmaceuticals, underscoring the broad impact of pyroglutamate analysis in diverse scientific domains. Creative Proteomics follows the continuous development and application of advanced technologies as we explore pyroglutamate formation in greater depth and advance new dimensions in both basic unclassified research and clinical applications.
Pyroglutamate Aminopeptidase I Promotes HepatocellularCarcinoma via IL-6/STAT3 Activation as Revealed by a SpecifificBiosensor.
Journal: Analytical Chemistry
Published: 2021
Background
Hepatocellular carcinoma (HCC) has a very high mortality rate as a malignant tumor. Numerous studies have shown that hepatocellular carcinoma and inflammation are closely related, which may be a key strategy in the diagnosis and treatment of hepatocellular carcinoma. Pyroglutamate aminopeptidase I (PGP-1) is a cysteine protease that removes terminal pyroglutamate from proteins or polypeptides, which is valuable for maintaining the metabolic homeostasis of proteins and polypeptides. Recent studies have found that PGP-1 is closely associated with inflammation generated in cells and tissues and is considered as a new inflammatory factor. Although the correlation between PGP-1 and many cancers has been reported, the mechanism of action is still unknown because of the lack of biosensors that can effectively track PGP-1.
Therefore, in this paper, the authors designed a dicyanoisophorone-based probe (DCID-pGlu) for detecting changes in intracellular PGP-1 levels and elucidating the correlation and mechanism of altered PGP-I levels with liver cancer progression. The authors found that knockdown of the PGP-I gene led to a significant reduction in the expression levels of the pro-inflammatory cytokines interleukin 6 (IL-6) and phosphorylated signal transducer and activator of transcription 3 (p-STAT3), which inhibited the proliferation and migration of hepatocellular carcinoma cells. The inhibition of HCC tumor growth by chamomile lactone may be attributed to chamomile lactone-mediated PGP-I inhibition. These findings suggest that PGP-I is a promising biomarker and a potential therapeutic target for HCC.
Results
First the authors determined that the probe DCID-pGlu could detect endogenous PGP-1 in the cells, as shown in Fig. 1a, the longer the probe incubation time, the more obvious the signal of the probe. In addition the authors pre-treated the cells with iodoacetamide, a common PGP-1 inhibitor, as shown in Figure 1c, the red fluorescence in HuH-7 cells significantly decreased with the concentration of iodoacetamide, and the red fluorescence in HuH-7 cells significantly decreased, indicating that the change in fluorescence was indeed induced by hydrolysis of endogenous PGP-I.
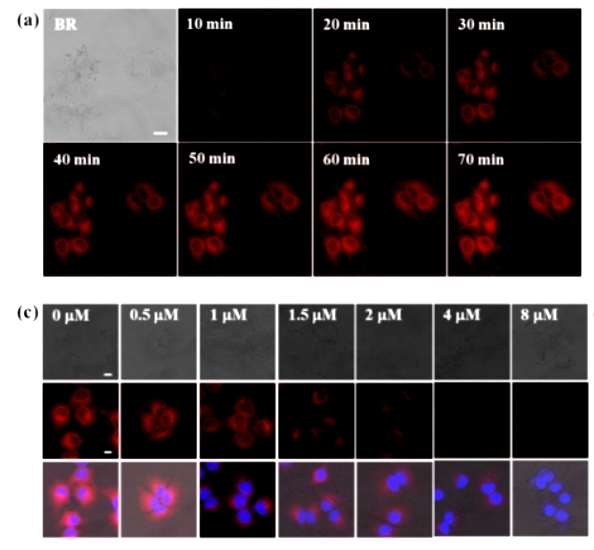 Figure 1
Figure 1
To quantify the dynamics of cellular PGP-I, PGP-I overexpression and knockdown were also performed to assess the sensing ability of DCID-pGlu. As shown in Figure 2a, b, the red fluorescence in HEK293 cells overexpressing rhPGP-I was much stronger than in Lipo control. In contrast, in HuH-7 cells, PGP-I knockdown (siRNA) resulted in weaker fluorescence emission than the RNAiMAX control. The authors then confirmed the overexpression of PGP-I in HEK293 cells by WB analysis, whereas the expression of PGP-I was reduced in siRNA-transfected HuH-7 cells (Figure 2c). These results indicated that DCID-pGlu could detect dynamic changes in PGP-I expression levels. Meanwhile, as shown in Figure 2d, PGP-I knockdown significantly inhibited the migration and propagation of HuH-7 cells.
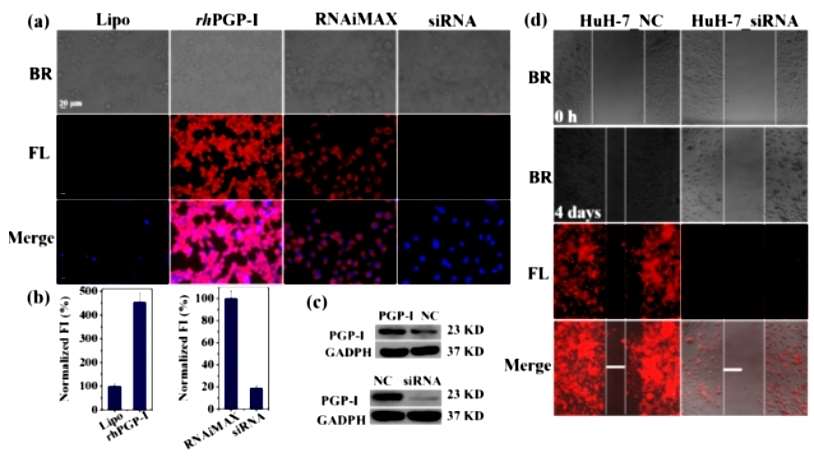 Figure 2
Figure 2
It has been demonstrated that IL-6 promotes the expansion of HCC stem cells through the STAT3 signaling pathway. When IL-6 levels are elevated in serum and tumor microenvironment, it may lead to STAT3 overactivation, especially STAT3 phosphorylation in tumor cells. Therefore, to understand the association between abnormal levels of PGP-I and the IL-6/STAT3 signaling pathway, the authors used WB to measure the true PGP-I content in HEK 293 cells overexpressing PGP-I (OE) and in PGP-I knockdown (siRNA) (Figure 3). IL-6 expression levels were significantly higher in OE compared to normal HEK 293 cells, while it was barely detectable in siRNA. Also, the amount of phosphorylated STAT3 was much higher in PGP-I overexpressing cells and significantly attenuated in PGP-I knockdown cells, suggesting that elevated PGP-I activated STAT3 phosphorylation and subsequent dimerization. Collectively, these results suggest that PGP-I may promote HCC by targeting the IL-6/STAT3 signaling pathway and may serve as a biomarker for HCC.
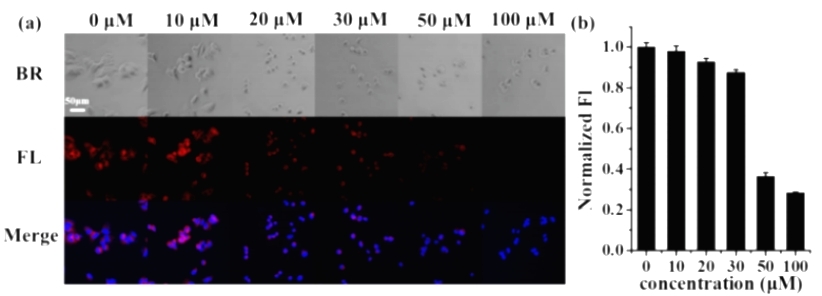 Figure 3
Figure 3
As a natural product, chamomile lactone has been reported to be a potent inhibitor of JAK activation, leading to a decrease in the phosphorylation status of STAT3. Therefore, chamomile lactone prevents STAT3 dimerization and thus prevents the growth of HCC cells. The authors pretreated the cells with chamomile lactone and then imaged them with DCID-pGlu, and found that the fluorescence signal decreased with the increase of chamomile lactone concentration. Therefore, the authors suggested that the antitumor effect of chamomile lactone may be mediated through the inhibition of PGP-I. These observations imply that PGP-I has great potential as a new target for HCC therapy.
Conclusion
The authors demonstrated that PGP-1 promotes HCC through activation of the IL-6/STAT3 signaling pathway, which can be significantly attenuated by chamomile lactone. These results suggest that PGP-I is a potential cancer biomarker and anticancer drug target, and the developed biosensor could serve as an ideal tool for PGP-I-related chemical biology studies and drug discovery.
Reference
Our products and services are for research use only.