
- Home
- PTMs Proteomics
- Proteomics Analysis of S-nitrosylation
Protein S-nitrosylation involves covalently linking nitric oxide (NO) molecule to the thiol group of a cysteine residue, leading to the formation of S-nitrosothiols that mediate redox-based signaling and primarily convey the general effects of NO on cellular function. Based primarily on liquid chromatography-tandem mass spectrometry (LC-MS/MS), Creative Proteomics can help researchers detect the degree of S-nitrosylation of proteins in cells and tissues under different physiopathological conditions and provide better results.
S-nitrosylation is a reversible covalent chemical reaction in which a NO is coupled to a critical cysteine thiolate (-S−) on a target protein, and finally generates an S-nitrosothiol. S-nitrosylation is achieved by triggering conformational changes in the protein that affect its activity, subcellular localization and interaction with partners. To date, over 3000 protein candidates have been identified that may undergo S-nitrosylation under normal and/or pathological conditions. Approaches such as the biotin-switch technique and MS-based proteomics have contributed greatly. S-nitrosylated proteins cover a wide range of cellular functions, such as receptors, metabolic enzymes, ion channels, signaling proteins, proteases, and structural proteins. In addition, the removal of NO from cysteine residues, i.e. denitrosylation, has been found to be a key mechanism for regulating protein activity, protein-protein interactions, and more generally signal transduction.
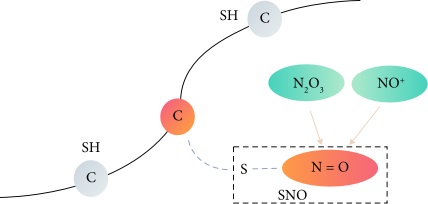 Fig. 1 A schematic
diagram of protein S-nitrosylation sites. (Zhao, Qian, et al., 2021)
Fig. 1 A schematic
diagram of protein S-nitrosylation sites. (Zhao, Qian, et al., 2021)
Increasingly, studies have focused on systematically analyzing the extent of protein S-nitrosylation in tissues and cells. We have established a highly sensitive HPLC-MS/MS pipeline that can help researchers better understand the mechanisms by which protein S-nitrosylation occurs. Typically, modified protein samples can be separated using SDS-PAGE, digested with sequence-specific proteases, and then analyzed in a mass spectrometer connected to a matrix-assisted laser desorption ionization (MALDI) or electrospray ionization (ESI) ionization source. Peptides obtained by enzymatic digestion can be further separated by liquid chromatography for easy identification. Relying on the mass-to-charge shift of a specific PTM, MS can be successfully used to detect and identify S-nitrosylation modifications. Our optimized method enables faster and more sensitive analysis of S-nitrosylated proteins.

As one of the important PTM of protein, S-nitrosylation regulates multiple biological processes by modulating protein activity, stability, subcellular localization, and protein-protein interactions. Exploration of the role of S-nitrosylation in human diseases can assist to clarify the pathogenesis and therapeutic strategies. Our customer service representatives are available 24 hours a day, 7 days a week. Please feel free to contact us for more details, and you will receive fast and professional assistance.
References
Our products and services are for research use only.