
- Home
- PTMs Proteomics
- Proteomics Analysis of Lactylation
Protein lysine lactylation is a novel post-translational modification (PTM) widely found in fungal and mammalian (e.g. human and mouse) cells that directly stimulates gene transcription and significantly affects downstream gene expression and DNA replication. Understanding the role and regulatory mechanisms of lysine lactylation in physiological and pathological processes that are highly dependent on glycolysis and lactate is essential. Creative Proteomics is committed to providing customized and highly effective protein lactylation analysis services and solutions to meet the specific needs of their projects.
Lactate is the end product of glycolysis and has long been recognized as a waste product that causes muscle fatigue and potential tissue damage. As lactate research continues to advance, it plays an important role not only as an intermediate in cellular metabolism but also as a signaling molecule for cellular communication. In general, protein lactylation is a novel PTM that has been found to modify lysine residues on histones in response to a range of cellular stresses (e.g. bacterial infection and hypoxia). The discovery of histone modification not only provides the molecular basis for lactate regulation of gene expression but also greatly expands the biological functions of lactate metabolism. In addition, it is reported that lysine lactylation is associated with a range of human diseases, including ischemic heart and brain diseases, bacterial infection, tumor development, and so on.
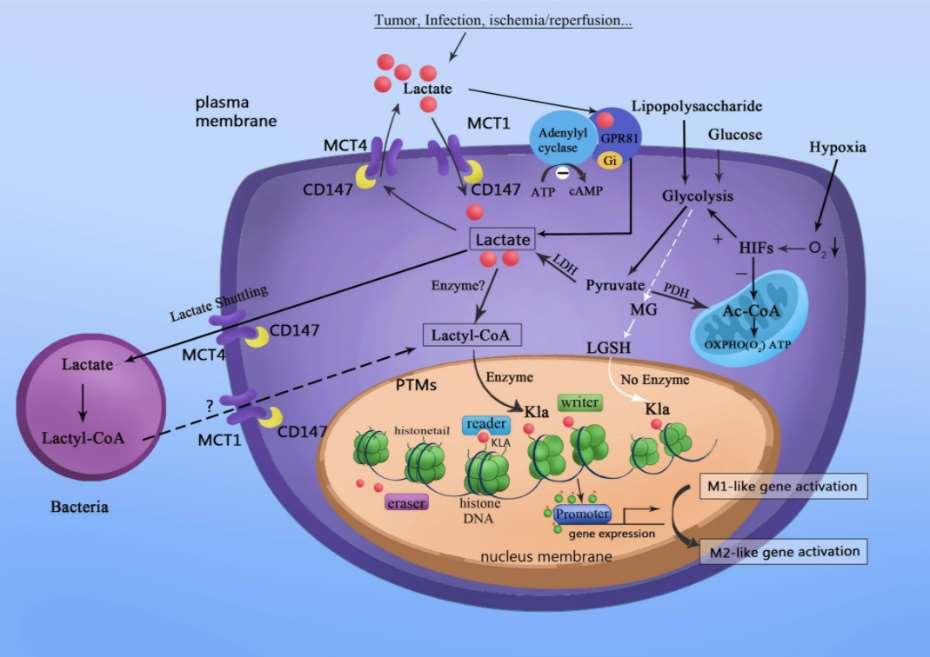 Fig.
1 Lactate acts as a signaling molecule to stimulate gene transcription via histone lysine lactylation in M1
macrophages. (Xin, Qi, et al., 2022)
Fig.
1 Lactate acts as a signaling molecule to stimulate gene transcription via histone lysine lactylation in M1
macrophages. (Xin, Qi, et al., 2022)
We have developed highly efficient mass spectrometry (MS) -based proteomics technology for system-wide identification and quantification of PTM substrates and mapping their sites. As for lysine lactylation analysis, we combine anti-lactate antibody-based immunoprecipitation with liquid chromatography-tandem mass spectrometry (LC-MS/MS) techniques. Our expert team focuses on using a range of enzymatic and chemical cleavage methods, as well as extensive fractionation, to maximize proteomic sequence coverage. In addition, our bioinformatics platform can contribute to the understanding of the functional relevance of protein lactylation in cellular physiology. Our services specifically include:
-Test the samples you provide before the official experiment.
-Protein extraction and digestion.
-Affinity enrichment of lysine lactylated peptides.
-MS data quality control.
-Lysine lactylated protein and site identification and analysis.
-Lactylated site pattern analysis.
-Lactylated protein cellular localization and functional enrichment analysis.
-Protein-protein interaction (PPI) network of lactylated proteins.

Contact us today to learn more about our lysine lactylation analysis services.
References
Our products and services are for research use only.