- Home
- Applications
- Protein Post-Translational Modifications in Biological Research
Post-translational modifications (PTMs) add a layer of complexity to the proteome by adding biochemical moieties to specific residues of proteins. To date, more than 600 types of PTMs have been identified. They are crucial for the normal functioning of the cells, and each modification has multiple effects on the proteins, involving their structure, function, and localization. PTMs also play key roles in a wide range of biological regulatory mechanisms, such as signaling pathways, protein-protein interactions, DNA damage response, and metabolic pathways.
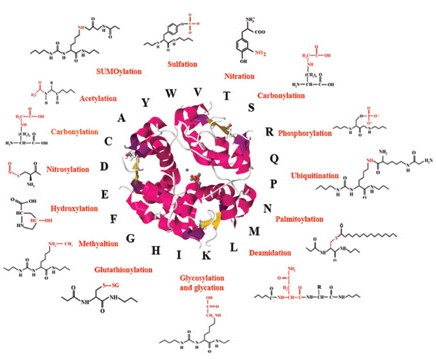 Fig.1 Post-translational modifications of proteins: biomarkers and therapeutic targets for diabetes related complications. (Vanuopadath, Muralidharan, et al, 2016)
Fig.1 Post-translational modifications of proteins: biomarkers and therapeutic targets for diabetes related complications. (Vanuopadath, Muralidharan, et al, 2016)
Phosphorylation is a reversible modification regulated by kinases and phosphatases. As one of the most important PTMs and the most extensively studied PTMs of proteins, phosphorylation is involved in a wide range of cellular functions. Phosphorylation has a vital role in cell signaling, transcriptional regulation, cell metabolism, apoptosis, immunological responsiveness, and other cellular processes. The disruption in the pathway of phosphorylation is related to a variety of diseases, such as neurodegenerative diseases, heart disease, and cancer.
Acetylation is one of the prominent PTMs that play an essential role in many cellular processes, including chromatin stability, protein-protein interactions, cell cycle control, cell metabolism, nuclear transport and actin nucleation. Acetylated lysine is essential for cell development and its dysregulation has been reported to be linked to cancer, aging, Huntington's chorea, Parkinson's disease, immune disorders, and cardiovascular disease. To date, acetylation can be divided into three forms, namely, α-acetylation, Nε-acetylation and O-acetylation.
Methylation is the process of transferring a carbon-methyl group to the N-terminal or o-terminal end of a target protein residue by methyltransferases (mainly lysine and arginine amino acids). Methylation often occurs in the cell nucleus and on the nuclear proteins and is a reversible PTM. Its most biologically important role is in histone modification. Moreover, methylation is associated with transcriptional regulation, signal transduction, and other vital biological functions. Defects in the methylation pathway can result in cancer, diabetes, and mental retardation.
Ubiquitination is a versatile PTM that can occur on all 20 amino acids, with more frequent ubiquitination of lysine. As one of the most important reversible PTMs, ubiquitylation plays a key role in a variety of cellular processes, including transcriptional regulation, signal transduction, proliferation, DNA repair, intracellular trafficking, and most notably, degradation of the protein. In addition, ubiquitination is involved in regulating stem cell differentiation and preservation by controlling stem cell pluripotency. Dysfunction in this modification can lead to many pathogeneses, including neurodegenerative disease, different cancers, diabetes, and immunological disorders.
Unlike ubiquitination, SUMOylation does not target proteins for protein hydrolysis, but rather regulates protein function in a variety of cellular processes, such as protein-protein interactions, protein stability, intracellular protein trafficking and localization. This modification plays a major role in transcription control, signal transduction, chromatin organization, macromolecule accumulation, gene expression, and other basic cellular processes. Also, accumulating evidence suggests that abnormal SUMOylation regulation is highly associated with diverse diseases, such as neurodegenerative diseases, heart diseases and diabetes, viral infections, and different cancers. In recent years, SUMOylation has become one of the hotspots of research as a competitor to ubiquitination.
Creative Proteomics is a leading custom service provider in PTM proteomics analysis. With a team of experts and state-of-the-art equipment, our PTM proteomics analysis platform helps our clients navigate successful research projects through robust workflows, robust analysis, and tailored services. For more information about our services, please feel free to contact us.
References
Our products and services are for research use only.