
- Home
- PTMs Proteomics
- Proteomics Analysis of Lysine β-hydroxybutyrylation
Lysine β-hydroxybutyrylation (Kbhb) has emerged as a pivotal post-translational modification, orchestrating cellular processes with finesse. This modification involves the addition of a β-hydroxybutyryl group to lysine residues, imparting a nuanced layer of regulation to diverse cellular functions. With implications reaching beyond metabolic pathways, Kbhb presents an intriguing landscape for exploration.
The biochemical intricacies underlying Kbhb reveal a complex interplay between enzymes and metabolites. Principally associated with cellular energy status, the levels of β-hydroxybutyrate, a ketone body, dictate the extent of lysine β-hydroxybutyrylation. This reversible modification is orchestrated by enzymes like lysine β-hydroxybutyryltransferases (KbhbTs) and lysine β-hydroxybutyrylhydrolases (KbhbHs), finely tuning the cellular response to nutrient availability.
The exploration of lysine β-hydroxybutyrylation (Kbhb) demands sophisticated analytical techniques capable of discerning this subtle post-translational modification within the complex cellular milieu. Cutting-edge methods have evolved, offering researchers a toolkit for in-depth investigation.
Bottom-up Proteomics. Bottom-up proteomics represents a cornerstone in Kbhb analysis. The process involves enzymatic cleavage of proteins into peptides, facilitating the identification of β-hydroxybutyrylated lysine residues. Following digestion, liquid chromatography coupled with high-resolution mass spectrometry allows for the sensitive detection and quantification of Kbhb peptides. This method provides a comprehensive overview of the Kbhb proteome, enabling researchers to map the modification on a global scale.
Top-down Proteomics. Advancements in mass spectrometry have paved the way for top-down proteomics in Kbhb studies. This approach analyzes intact proteins, offering a unique perspective on the combinatorial nature of β-hydroxybutyrylation. With the ability to resolve multiple lysine residues within a single protein, top-down proteomics contributes to a more nuanced understanding of Kbhb heterogeneity.
Kbhb-Specific Antibodies. Leveraging the specificity of antibodies tailored for β-hydroxybutyrylated lysine residues has emerged as a targeted approach in Kbhb research. Immunoprecipitation using these antibodies, followed by mass spectrometry or Western blotting, allows for the selective isolation and analysis of Kbhb-modified proteins. This targeted strategy proves invaluable in studying Kbhb dynamics in specific cellular contexts.
Immunohistochemistry (IHC) and Immunofluorescence (IF). For spatial insights into Kbhb, IHC and IF techniques utilizing Kbhb-specific antibodies provide a means to visualize the subcellular localization of this modification. This spatial resolution is particularly crucial when exploring Kbhb's role in organelle-specific processes or elucidating its involvement in cellular signaling cascades.
Lysine β-hydroxybutyrylation stands as a testament to the intricacies of cellular regulation. From analytical challenges to diverse applications, the journey into Kbhb research is marked by continuous innovation and a deepening appreciation for the subtleties that govern cellular function. Creative Proteomics is dedicated to the analysis of protein modifications, and our integrated use of advanced technological approaches has played an important role in helping researchers deepen their understanding of Lysine β-Hydroxybutyrylation.
Ketogenesis Impact on Liver Metabolism Revealed by Proteomics of Lysine β-hydroxybutyrylation
Journal: Cell Rep
Published: 2021
Background
In this study, specific antibodies and β-hydroxybutyryl modification histology were used to examine the changes in protein Kbhb levels in mouse liver and kidney with different β-OHB contents. Based on this study, it was found that β-hydroxybutyryl modification directly inhibited the activity of the key rate-limiting enzyme of the methionine cycle, S-adenosyl-L-homocysteine hydrolase (AHCY), which further revealed the roles of β-hydroxybutyryl modification in physiological and pathological processes.
Results
The researchers first detected the Kbhb levels of total proteins in various tissues of mice using a β-hydroxybutyryl-modified pan-antibody, and found that the blood levels of β-OHB were significantly elevated in the mice after 48 hours of fasting, along with enhanced levels of liver proteins, and then returned to the initial levels of the fasting period after re-feeding the mice, and similarly, kidney proteins Kbhb was also was significantly enhanced after fasting. However, pancreatic, skeletal muscle, rectal, cerebral cortex, and cardiac protein Kbhb were not significantly altered by starvation stress, suggesting that the effect of β-OHB on protein Kbhb is tissue-specific. In addition, the whole protein Kbhb levels of in vitro cell lines (mouse HEP1C, MEF, and human HEK293T) were also enhanced with increasing concentrations of β-OHB (Figure 1).
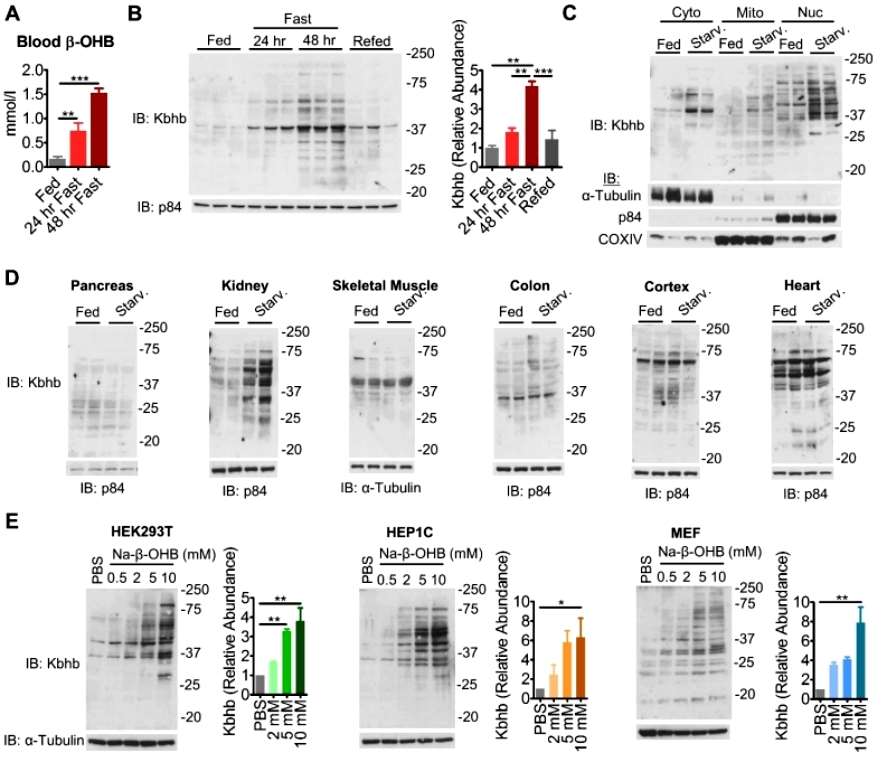 Figure 1
Figure 1
Ketogenesis produces primarily β-OHB, and it was then theorized that any other physiological/pathological state that promotes ketone body production would cause an enhancement of the protein Kbhb. So, the researchers fed the mice a ketogenic diet in two ways: 1. a diabetogenic dose of streptozotocin was injected to promote ketogenesis in the mice. Indeed, both of these approaches resulted in a significant increase in β-OHB levels in the blood of the mice, which in turn caused enhanced levels of the liver and kidney protein Kbhb. Importantly, similar to the starvation stress results, the ketogenic diet also only tissue-specifically promoted liver and kidney protein Kbhb levels (Figure 2).
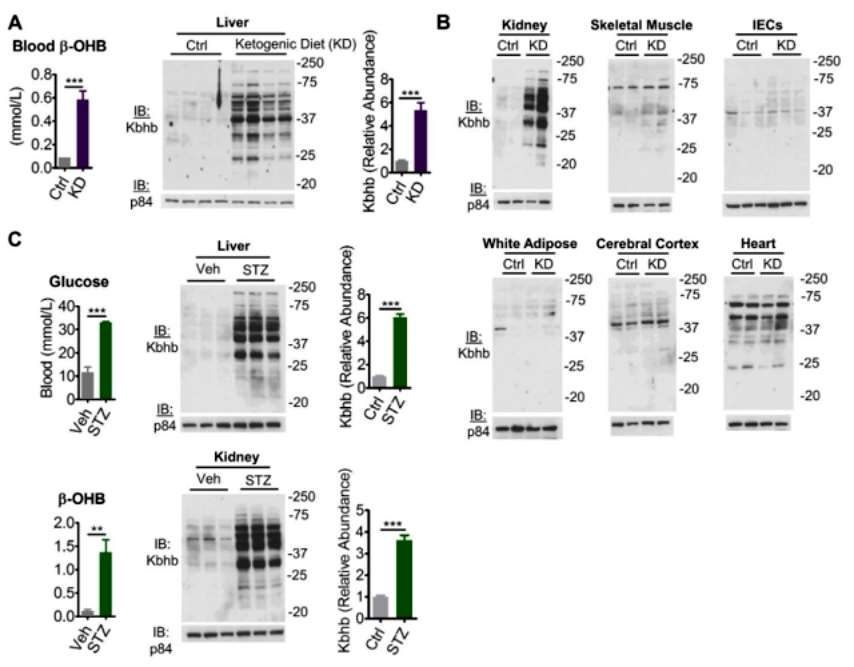 Figure 2
Figure 2
Next, the researchers detected a total of 267 proteins with a total of 891 lysine sites undergoing β-hydroxybutyrylation modification in starved mouse livers (sample strategy) using β-hydroxybutyrylation modification histology (mass spectrometry strategy), which, 82.8% of the proteins and 72.4% of the lysine sites could also undergo acetylation modification. Subcellular localization analysis showed that β-hydroxybutyrylation-modified proteins were widely distributed in the cytoplasm, mitochondria, and nucleus, implying the extensive effects of β-hydroxybutyrylation modification on cellular functions. Through modification site analysis, the researchers found that 114 proteins (42.7%) had only one Kbhb site, however, many key metabolic enzymes have upwards of 10 Kbhb sites. For example, the urea cycle rate-limiting enzyme CPS1 and the ketogenesis rate-limiting enzyme HMGCS2 contain 35 and 15 Kbhb sites, respectively.GO analysis also revealed that Kbhb is enriched in many proteins in core hepatic metabolic pathways, including the three major nutrient metabolisms, glutathione metabolism, one-carbon metabolism, and others (Figure 3).
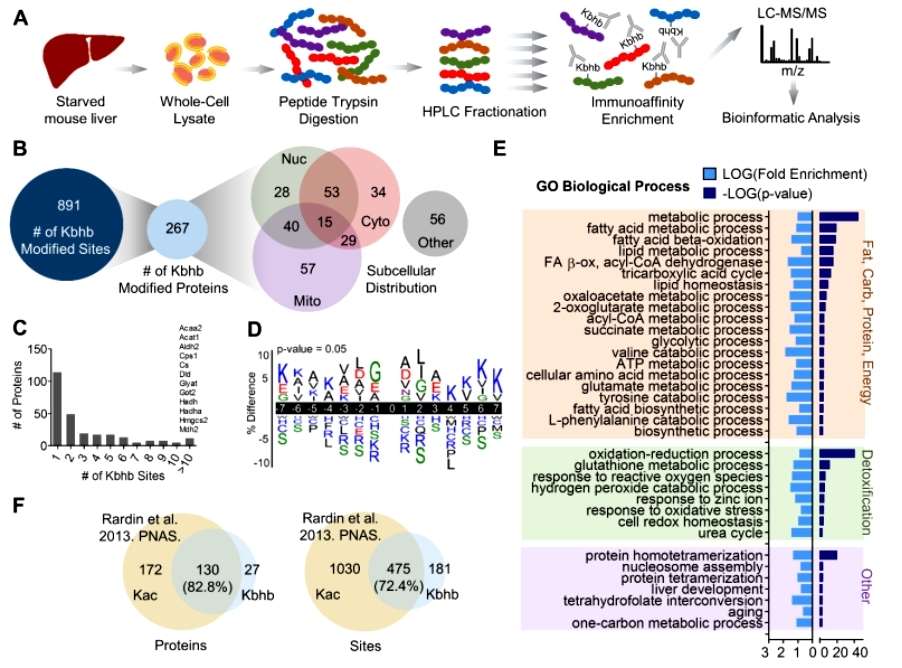 Figure 3
Figure 3
Interestingly, the researchers noted that Kbhb occurred in numerous metabolic enzymes involved in methionine metabolism, so they next focused on the effect of β-OHB on the activity of AHCY, the hydrolase that catalyzes the production of homocysteine (HCY) from S-adenosyl methionine (SAH). After overexpressing AHCY in MEF cells, the researchers found that AHCY Kbhb levels were significantly enhanced after 8 hours of Na-β-OHB incubation using a specific antibody, and in addition, both starvation stress and a ketogenic diet enhanced AHCY Kbhb. Importantly, by assaying substrate concentrations to characterize AHCY activity, the researchers found that 8 hours of Na-β-OHB incubation also had the greatest inhibitory effect on AHCY activity. AHCY activity was also most significantly inhibited by an 8-hour incubation with Na-β-OHB, suggesting that β-OHB can inhibit AHCY activity through β-hydroxybutyrylation modification. Subsequently, by analyzing the structure of AHCY and the mutation of the Kbhb site, the researchers confirmed that Kbhb could inhibit AHCY activity by affecting the spatial conformation of the NAD+ binding site of AHCY (Figure 4).
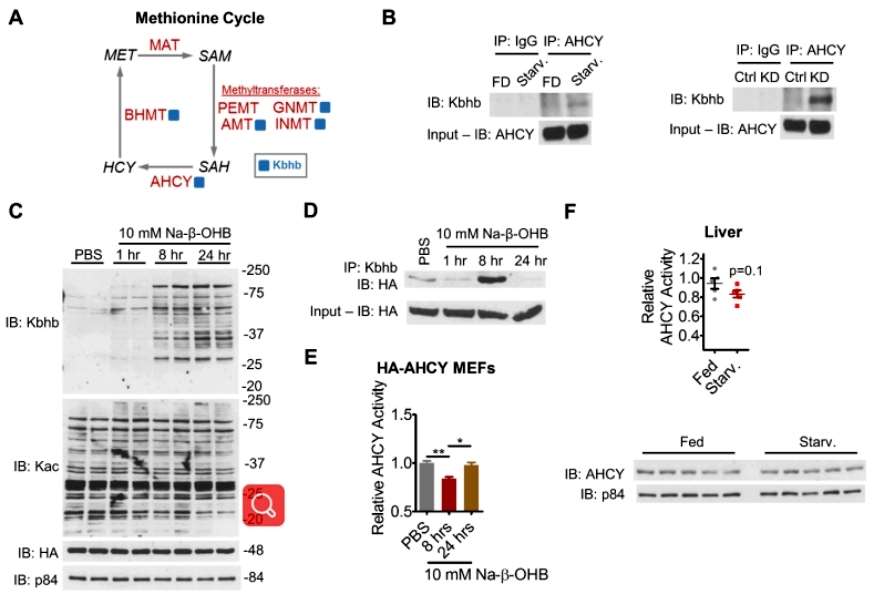 Figure 4
Figure 4
Conclusion
In this paper, the researchers used β-hydroxybutyrylation modification-specific pan-antibodies to systematically study for the first time the multiple organ/tissue protein Kbhb profiles in mice, and investigated the effects of β-OHB on protein Kbhb in physiological and pathological states using various methods to promote ketogenic effects, such as starvation stress and ketogenic diet. Importantly, using β-hydroxybutyrylation modification histology techniques, the researchers also found that Kbhb is enriched in a variety of key hepatic metabolic processes, and combined with structural analyses, and site-specific mutations, the inhibitory effect of Kbhb on AHCY was demonstrated in detail. Considering that tumor cell proliferation is highly dependent on one-carbon metabolic processes and that AHCY has been reported as a potential target for tumor therapy. Therefore, in combination with the evidence from this study, it is likely to imply that the level of AHCY Kbhb modification can be enhanced in the future by increasing the level of β-OHB in vivo through a ketogenic diet, in order to serve as an inhibitor of methionine metabolism and inhibit the proliferation of tumors.
Reference
Our products and services are for research use only.